| Monthly Tech-Tip | No tracking! No ads! |
Cone 6 transparent way better without Gerstley Borate.
I surgically removed it to create G2926B!
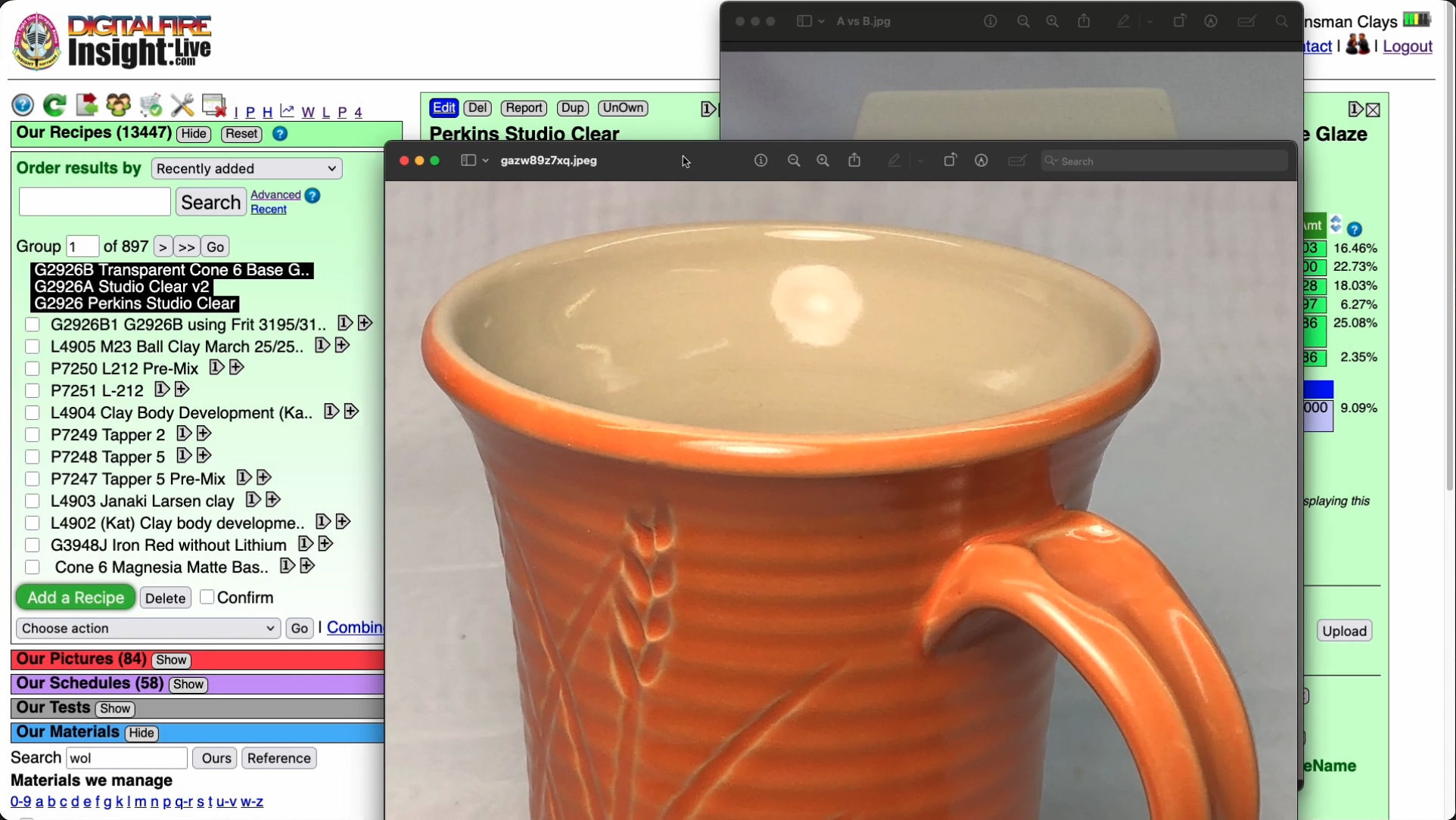
These are the original cone 6 Perkins Studio Clear (left) beside our fritted version (right). You cannot just substitute a frit for Gerstley Borate (GB), they have very different chemistries. But, using the calculation tools in my account at insight-live.com, I compensated for the differences by juggling other materials in the recipe. I even upped the Al2O3 and SiO2 a little on the belief they would dissolve in the more active melt the frit would create. I was right - a melt-flow GLFL test comparison (inset left) shows that the GB version flows less. Using this on ware exhibited another issue (after doing a IWCT test): Crazing. The very good melt flow on my G2926A fritted version is thus good news: It can accept more silica - the more silica, the more durable and craze resistant it will be. How much did it take? 10% more! That ultimately became the recipe for our standard G2926B cone 6 transparent.
Related Pictures
Click here for case-studies of Insight-Live fixing problems

This picture has its own page with more detail, click here to see it.
You will see examples of replacing unavailable materials (especially frits), fixing various issues (e.g. running, crazing, settling), making them melt more, adjusting matteness, etc. Insight-Live has an extensive help system (the round blue icon on the left) that also deals with fixing real-world problems and understanding glazes and clay bodies.
Somehow the Gerstley Borate 50:30:20 glaze worked.
But does it work using Gillespie Borate?

This picture has its own page with more detail, click here to see it.
This recipe, G2826A, a base transparent recipe having 50% Gerstley Borate plus 20% kaolin, is "jelly city". Although a low temperature base, this was much more commonly used at cone 5-6. This recipe, G2826A, was at the limit of how melt fluid a glaze could be. And at the limit of the slurry properties that could be tolerated with this material. In this test, even with 2.5g of Darvan deflocculant in this jar, it was still thick enough to require pushing this tile down into it! It still needed 5 seconds to build up enough thickness. And did not cover the recesses properly. Yet people have used this popular fluid-melt recipe for 50+ years to get the surface variegation it produces (because of boron blue) and the fluid melt (because it is so high in boron). They added all manner of colorants and opacifiers and it generally performed without blistering. The melt fluidity required careful control of thickness (to avoid it running onto shelves). This was a "Dr. Jekyll and Mr. Hyde" of ceramic materials!
Potters are using Gillespie Borate in this recipe (with issues), see the G2826A2 recipe. Other approaches are to source the boron (B2O3) from a frit (or mix of frits). An example is G2826A1, it does not variegate as much but added titanium or rutile can emulate that. Another hybrid option is the G2826A3 that employs both Gillespie Borate, nepheline and talc.
Videos
 |
How I Developed the G2926B Cone 6 Transparent Base Glaze
How I found a pottery glaze recipe on Facebook, substituted a frit for the Gerstley Borate (using glaze chemistry), compared using a melt flow tester, added as much extra SiO2 as it would tolerate, and got a durable and easy-to-use cone 6 clear. |
Links
| Articles |
Trafficking in Glaze Recipes
The trade is glaze recipes has spawned generations of potters going up blind alleys trying recipes that don't work and living with ones that are much more trouble than they are worth. It is time to leave this behind and take control. |
| Articles |
A Low Cost Tester of Glaze Melt Fluidity
Use this novel device to compare the melt fluidity of glazes and materials. Simple physical observations of the results provide a better understanding of the fired properties of your glaze (and problems you did not see before). |
| Glossary |
Glaze Chemistry
Glaze chemistry is the study of how the oxide chemistry of glazes relate to the way they fire. It accounts for color, surface, hardness, texture, melting temperature, thermal expansion, etc. |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy

