| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
A lead bisilicate frit fails a leach test. Yet as-a-glaze it passes. Why?
I have soaked a lead bisilicate frit in vinegar overnight. To test whether it is leaching I pour the vinegar leachate into a test tube, soak a Q-Tip in the sensor solution and dip it into the vinegar. It turns black immediately - so we have lead in the leachate! But this is not as it seems.
Remember a key point here: The frit glass had no opportunity to be annealed - it was crash-cooled by being quenched in water. Annealing and associated toughening of the surface is a natural consequence of a glaze cooling slowly in a periodic kiln - which is why pieces made using an 85:15 mix of this same frit and kaolin, pass this test. The same 85:15 mix also still passes a lead check test if melted into an ingot and crushed into a granular powder (this is amazing given the exponential increase in surface area).
Related Pictures
Lead bisilicate with his ugly borosilicate cousins at a cone 05 party

This picture has its own page with more detail, click here to see it.
The middle front mug is glazed with an 85:15 lead bisilicate:kaolin mix, the G3971 recipe. It is an absolutely "knock your socks off" crystal-clear hyper-glossy surface that transmits the terra cotta color beautifully regardless of whether the clay is smooth or coarse or the glaze thick or thin (this one was applied as a brushing glaze in three coats on L215). My lead testing kit passes it with no detectable lead release. The other pieces are done using brush-on versions of boron-based clear glazes (commercial and made from a recipe). At almost any thickness and whether on L215 or L4170B clouding occurs. The worst one is a commercial three-coater on the right, the best is G1916W (it has 2% added iron as a fining agent for the micro-bubbles). My terra cotta plan: Glaze the inside functional surfaces with that and the outsides with the leaded one (and using a kiln exhaust system).
Lead bisilicate frit data sheet claims high resistance to leaching
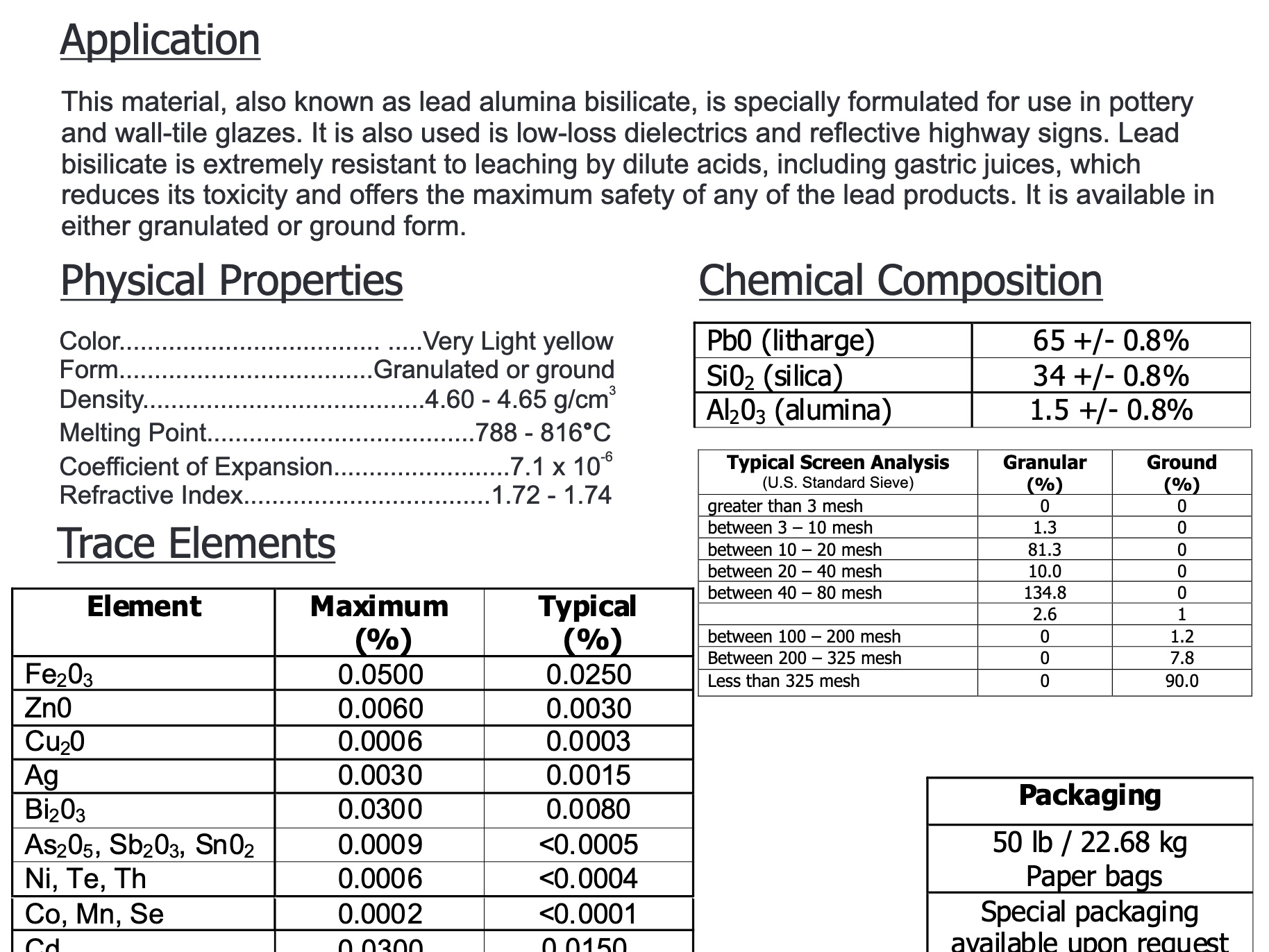
This picture has its own page with more detail, click here to see it.
Notice it is specifically "formulated for use in pottery glazes". And that "Lead bisilicate is extremely resistant to leaching by dilute acids, including gastric juices, which reduces its toxicity and offers the maximum safety of any of the lead products." In many countries, the use of lead glazes is still considered normal and safe. There is zero use of lead in pottery glazes in North America. Is it possible that we have tarred all lead products with the same brush? Could we at least be using it on non-functional decorated surfaces of low-fire stoneware? This would drastically reduce the energy consumption of kilns.
Videos
Links
| Materials |
Lead Bisilicate Frit
A standard frit of 1 molar part of PbO and 2 of SiO2. It is considered stable and non-leachable. |
| Glossary |
Lead in Ceramic Glazes
Lead is a melter in ceramic glazes and performs exceptionally well and must be misused to be toxic. It is also now environmentally pervasive. It is toxic and cumulative at any level of exposure. |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
