| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Gerstley Borate
Alternate Names: Colemanite, Calcium Borate, Borocalcite
Description: Plastic Calcium Borate
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 19.40% | 0.69 | |
| K2O | 0.40% | 0.01 | |
| MgO | 3.50% | 0.17 | |
| Na2O | 4.00% | 0.13 | |
| B2O3 | 26.80% | 0.77 | |
| Al2O3 | 1.00% | 0.02 | |
| SiO2 | 14.80% | 0.49 | |
| Fe2O3 | 0.40% | - | |
| P2O5 | 0.10% | - | |
| TiO2 | 0.10% | - | |
| LOI | 29.50% | n/a | |
| Oxide Weight | 140.40 | ||
| Formula Weight | 199.15 | ||
Notes
March 2023: Gerstley Borate has just tripled in price. It’s demise is imminent. A search at lagunaclay.com does not return any hits for the term.
If you are seeking to replace this material in your recipes, please check the bottom of this page for links.
No common natural ceramic material in North America comes close to melting like Gerstley Borate (GB). It begins to melt between 1550F and 1600F and is a clear amber glass by 1750F and ultraclear and glossy by cone 06 (Ulexite melts better but it is not commonly in use in ceramics). It has thus been a staple among potters for many years. 50% or more can be found in many cone 06-02 glazes and 30% is common in cone 6 glazes. Gerstley Borate is also very plastic and thus suspends and hardens glazes as they dry. In fact, few clays have the plasticity and the ability to retain water that GB has. A GB slurry can take many hours to dewater on a plaster batt, even in a very thick layer. Thus it is common to find Gerstley-Borate-based recipes having no other clay content. Unfortunately many also have clay (e.g. Kaolin, ball clay, bentonite) and lots of GB so they shrink and crack on drying.
GB natural source of boron that was mined in southern California for many years. Mineralogically it is a combination of colemanite, ulexite and high plasticity clay (likely hectorite). The melting behavior of ulexite and colemanite is quite different, the unusual early melting behavior GB exhibits this, it suddenly implodes to a brown opaque melt (because of the earlier fluxing of ulexite) which later turns transparent (when the colemanite joins in).
The mine was closed in 2000 and remaining stocks were to be depleted in 2-3 years. There was alarm across the ceramic community in North America leading up to and after the closure (because Gerstley Borate formed the basis of so many glazes). However, in June 2011, the supplier, Lagunaclay.com, announced that there was again a large supply still available (from an unused stockpile). That lasted until 2022 when rumours arose that it would become permanently unavailable.
Prior to, and during the decade of uncertainty about the future of this material, the supplier did not provide updated chemistry information. It was during this time that many companies promoted substitutes. We rationalized it as 24% CaO, 4% MgO, 0.5% K2O, 4% Na2O, 2% Al2O3, 25% B2O3, 14% SiO2, 0.5% Fe2O3 and 26% LOI. In June 2011 we changed the chemistry provided here to the one provided by Laguna on their website (rounded to 1 decimal). This new chemistry has more B2O3 and less CaO (other oxide amounts are fairly similar).
Since GB glazes melt well and are so easy to make, most people have overlooked issues surrounding their use. Glazes with high GB content that host potentially toxic metallic colorants or other materials are often assumed to be non-leachable because they melt well (whereas, in fact, they may have unbalanced chemistry). Gerstley Borate has almost no Al2O3, this is a problem because glazes need it and Al2O3 is normally sourced from clays, especially kaolin. But since GB is so plastic, adding more plastic materials to a glaze causes excessive drying shrinkage (producing cracks and ultimately crawling). One solution is to use calcined kaolin. Another option is to source Al2O3 from feldspar, however, to get enough to create a stable glass oversupplies KNaO and causes crazing.
High GB glazes often have a lot of micro-bubbles in the fired glass and micro-dimples on the fired glaze surface (most visible in transparents). GB typically flocculated and gelled slurries, the greater the percentage the worse the problem was, this made even application difficult, slowing drying and resulting in cracks that often turned to crawling during firing. One common low to middle fire transparent, for example, had 50% GB and added 30% kaolin to that, producing an impossible-to-use slurry that potters somehow endured! This being said, some potters claimed trouble-free slurries of relatively high specific gravities having up to 30% of the material, it appears other ingredients in their recipes helped to deflocculate them.
Of course, the lower the percentage of GB in a glaze recipe the more practical it is to just replace it with a substitute (like Gillespie Borate). But when the proportion gets higher the best approach to finding an alternative is the use of glaze chemistry to substitute other material to source the B2O3 or boron (eligible frits also supply CaO and SiO2). This can be done in an account at Insight-live.com. Frits are less volatile, more consistent and reliable and do not flocculate or gel the glaze as Gerstley Borate does. No available frit contains as much B2O3 as does GB, this is some cases it is not possible to source sufficient B2O3. However, in the majority of such cases, the glaze was unbalanced and contained too much boron anyway.
If you need to substitute it in your glazes and need help, just purchase a group account at Insight-live.com and we can work together to get it done. You will end up with a glaze having better slurry properties, fewer bubbles and better fit.
Related Information
Gerstley Borate is a volatile melting material

This picture has its own page with more detail, click here to see it.
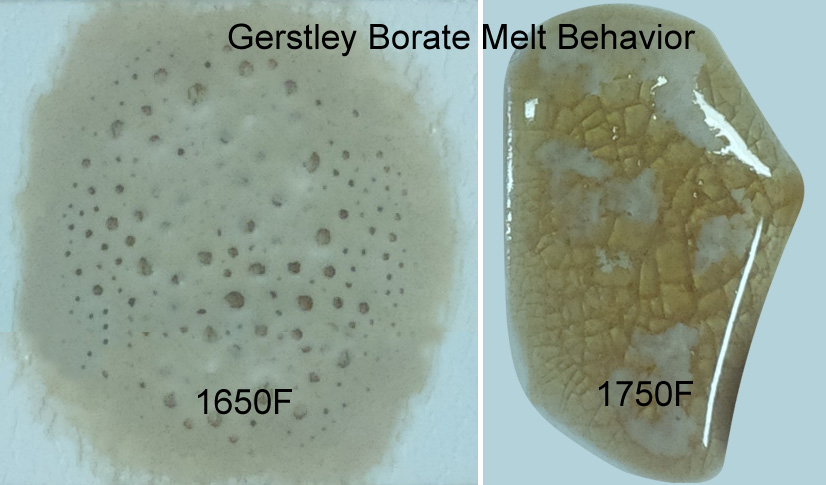
Gerstley Borate (compared with Ferro frit 3124) from 1600-1750F. At 1550F (not shown) it suddenly shrinks to a small ball and then by 1600F it has expanded to double its size. By 1650 it is well melted, but still gassing and bubbling.
Why does Gerstley Borate melt in two stages? Because it is two minerals.
This picture has its own page with more detail, click here to see it.
The ulexite in Gerstley Borate melts first, producing an opaque fired glass having the unmelted (and still gassing) particles of colemanite suspended in it. By 1750F the colemanite is almost melted also. Boron-containing frits, by contrast, soften slowly over a wide temperature range and gradually spread and melt. Not surprisingly they produce a more stable glaze (albeit often less interesting visually without additives e.g. titanium, rutile). These behavior contributes to phase changes in fired glazes that contribute to variegation.
Gerstley Borate official composition page from Laguna Clay

This picture has its own page with more detail, click here to see it.
We have rounded these numbers off for inclusion as a material used in glaze chemistry at Insight-live.com. This document was available as of July 2022, but as of April 2023 it is no longer there.
Can you actually throw a Gerstley Borate glaze? Yes!

This picture has its own page with more detail, click here to see it.
G2931 Worthington Clear is a popular low to medium-fire transparent glaze recipe. It contains 55% Gerstley Borate (GB) plus 30% kaolin (GB melts at a very low temperature). GB is also very plastic, like a clay. I have thrown a pot from this glaze recipe! This explains why high Gerstley Borate glazes often dry so slowly and shrink and crack during drying. When recipes also contain a plastic clay like this one the shrinkage is even worse. GB is also slightly soluble, over time it gels glaze slurries even in smaller percentages. Countless potters struggle with Gerstley Borate recipes.
At 1550F Gerstley Borate suddenly shrinks!

This picture has its own page with more detail, click here to see it.
These GBMF test balls were fired at 1550F and were the same size to start. The Gerstley Borate has suddenly shrunk dramatically in the last 40 degrees (and will shrink even more and then suddenly melt down flat within the next 50). The talc is still refractory, the Ferro Frit 3124 slowly softens across a wide temperature range. The frit and Gerstley Borate are always fluxes, the talc is a flux under certain circumstances.
The difference between these low fire transparents: Gerstley Borate vs. Ulexite

This picture has its own page with more detail, click here to see it.
Left: Worthington Clear cone 04 glaze (A) uses Gerstley Borate to supply the B2O3 and CaO. Right: A substitute using Ulexite and 12% calcium carbonate (B). The degree of melting is the same but the gassing of the calcium carbonate has disrupted the flow of B. Gerstley Borate gasses also, but does so at a stage in the firing that does not disrupt this recipe. However, as a glaze, B does not gel and produces a clearer glass. A further adjustment to source CaO from non-gassing wollastonite would likely improve it.
One reason why Gerstley Borate can make glaze difficult to use

This picture has its own page with more detail, click here to see it.
This glaze slurry contains 30% Gerstley Borate. I poured it onto a plaster table and it can take five or ten minutes to dewater enough to form it in test balls. A typical glaze would dewater twenty times faster! Gerstley Borate contains microfine clay (e.g. hectorite, bentonite), the percentage is high enough that it voraciously hangs on to the water it has. This is the reason that many GB glazes take a long time to dry on bisque ware. And why they require more water and gel the slurry. Generally, slow drying also means cracking, that in turn can lead to crawling.
Why would a glaze turn into a jelly like this?

This picture has its own page with more detail, click here to see it.
This is one of the things Gerstley Borate commonly does (when its percentage is high enough). It is also highly thixotropic - this can be stirred vigorously to thin it, yet within seconds it turns back to jelly again. This is part of the reason it is often referred to as “ghastly borate”. Side effects of this include high water content, slow drying, excessive shrinkage on drying, cracking and crawling. To attempt a fix, I deflocculated it with Darvan. It was stable enough to dip bisque ware, with difficulty (but pieces dried very slowly). But overnight it has turned back into a gel. What can be done with a mess like this? Start over, with three options:
1. Identify the mechanism to isolate the base recipe and substitute another.
2. Replace the Gerstley Borate with an equivalent that does not do this (Gillespie Borate).
3. Do a little chemistry to source the B2O3 from a frit instead (e.g. in an account at insight-live.com).
The Gerstley Borate 50:30:20 glaze was a bummer
Is the Gillespie Borate version any better?

This picture has its own page with more detail, click here to see it.
This recipe, G2826A, a base transparent recipe having 50% Gerstley Borate plus 20% kaolin, is "jelly city". Although a low temperature base, this was much more commonly used at cone 5-6. This recipe, G2826A, was at the limit of the slurry properties that could be tolerated with this material. In this test, even with 2.5g of Darvan deflocculant in this jar, it was still thick enough to require pushing this tile down into it! It still needed 5 seconds to build up enough thickness. And did not cover the recesses properly. Yet people have used this popular fluid-melt recipe for 50+ years to get the surface variegation its high melt fluidity produces (because it is so high in boron). They added all manner of colorants and opacifiers and it generally performed without blistering. This was a "Dr. Jekyll and Mr. Hyde" of ceramic materials!
Potters are using Gillespie Borate in this recipe (with issues), see the G2826A2 recipe. Other approaches are to source the boron (B2O3) from a frit (or mix of frits). An example is G2826A1, it does not variegate as much but added titanium or rutile can emulate that. Another hybrid option is the G2826A3 that employs both Gillespie Borate, nepheline and talc.
Glaze recipes online waiting for a victim to try them!

This picture has its own page with more detail, click here to see it.
You found some recipes. Their photos looked great, you bought $500 of materials to try them, but none worked! Why? Consider these recipes. Many have 50+% feldspar/Cornwall/nepheline (with little dolomite or talc to counteract their high thermal expansion, they will craze). Many are high in Gerstley Borate (it will turn the slurry into a bucket of jelly, cause crawling). Others waste high percentages of expensive tin, lithium and cobalt in crappy base recipes. Metal carbonates in some encourage blistering. Some melt too much and run onto the kiln shelf. Some contain almost no clay (they will settle like a rock in the bucket). A better way? Find, or develop, fritted, stable base transparent glossy and matte base recipes that fit your body, have good slurry properties, resist leaching and cutlery marking. Identify the mechanisms (colorants, opacifiers and variegators) in a recipe you want to try and transplant these into your own base (or mix of bases). And use stains for color (instead of metal oxides).
A cure for long-time Gerstley Borate sufferers

This picture has its own page with more detail, click here to see it.
These are various different terra cotta clays fired to cone 04 with a recipe I developed that sources the same chemistry as the popular G2931 Worthington clear (50:30:20 GB:Kaolin:Silica) but from a different set of materials. The key change was that instead of getting the B2O3 from Gerstley Borate I sourced it first from Ulexite (G2931B) and then from a mix of frits (G2931K). All pieces were fired with a drop-and-hold firing schedule C03DRH. Fit was good on many terra cottas I tried (pieces even surviving boiling:icewater stressing). Where it did not fit I had thermal expansion adjustability because more than one frit was sourcing the boron. Frits are so much better for sourcing B2O3 than Gerstley Borate (the latter is notorious for turning glaze slurries into jelly!). Of course, a little glaze chemistry is needed to figure out how to convert a recipe from Gerstley Borate bondage to frit freedom, but there is lots of information here on how to do that.
A good example of the superiority of a frit

This picture has its own page with more detail, click here to see it.
Both of these glazes were made as 1000 gram batches and then mixed with the necessary amount of water to produce a slurry of the correct consistency. The one on the left is a fritted glaze with 20% kaolin, the one on the right is a Gerstley Borate based raw glaze (30% GB + feldspar, silica, ball clay). The GB glaze required much more water and gelled shortly after (it also tends to dry slowly and crack during drying on the ware). The fritted glaze has very good slurry and application properties.
LOI horse race with surprising winners
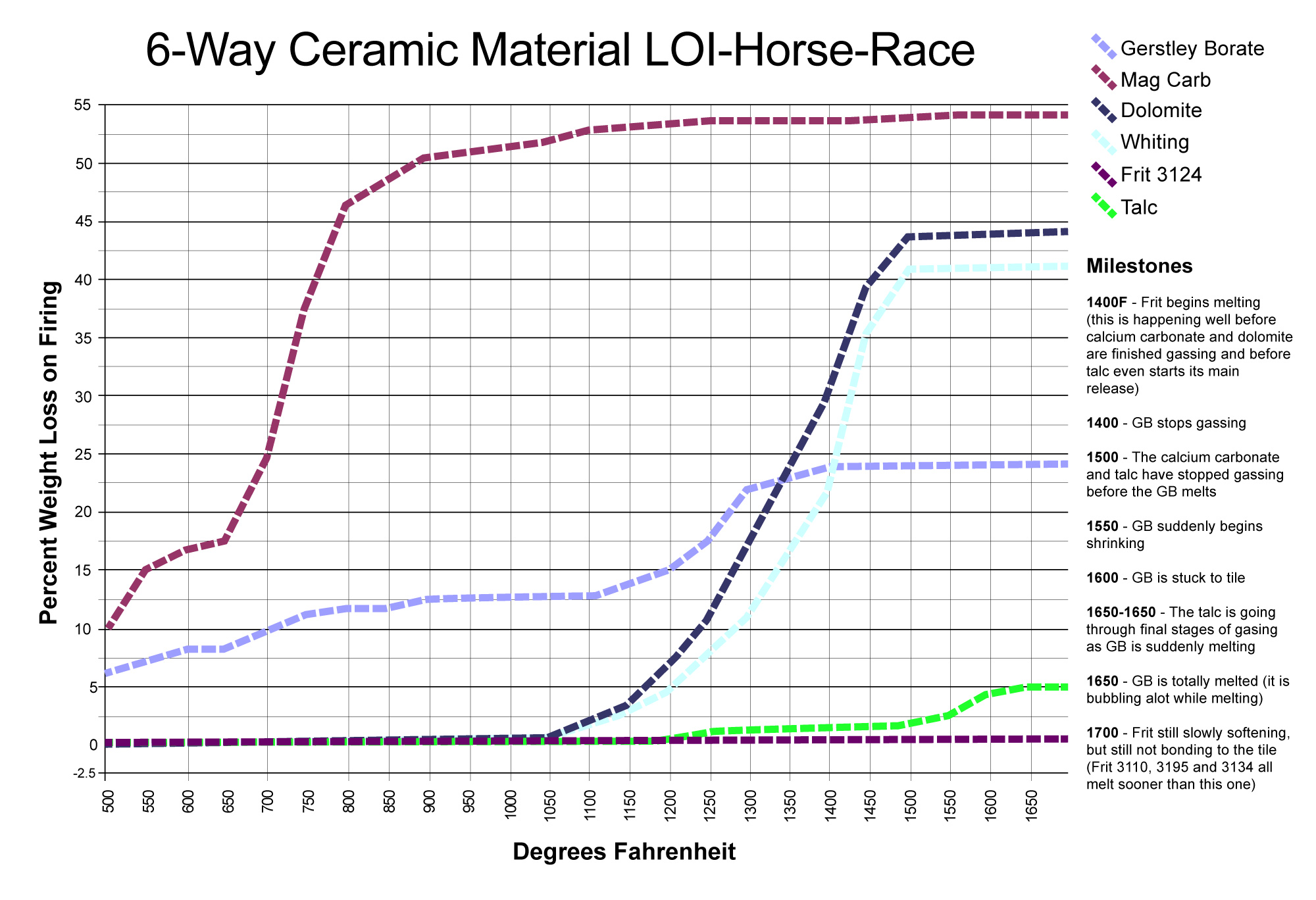
This picture has its own page with more detail, click here to see it.
This chart compares the decompositional off-gassing (% weight Loss on Ignition) behavior of six glaze materials as they are heated through the range 500-1700F. It is amazing how much weight some can lose on firing - for example, 100 grams of calcium carbonate generates 45 grams of CO2! This chart is a reminder that some late gassers overlap early melters. That is a problem. The LOI of these materials can affect glazes (causing bubbles, blisters, pinholes, crawling). Talc is an example: It is not finished gassing until 1650F, yet many fritted glazes have already begun melting by then. Even Gerstley Borate, a raw material, begins to melt while talc is barely finished gassing. Dolomite and calcium carbonate Other materials also create gases as they decompose during glaze melting (e.g. clays, carbonates, dioxides).
Comparing glaze melt fluidity balls with their chemistries

This picture has its own page with more detail, click here to see it.
Ten-gram GBMF test balls of these three glazes were fired to cone 6 on porcelain tiles. Notice the difference in the degree of melt? Why? You could just say glaze 2 has more frit and feldspar. There is a better explanation, compare these yellow and blue numbers: Glaze 2 and 3 have much more B2O3 (boron, the key flux for cone 6 glazes) and lower SiO2 (silica, it is refractory). But notice that glaze 2 and 3 have the same chemistry, but 3 is melting more? Why? Because of the mineralogy of Gerstley Borate. It release its boron earlier in the firing, getting the melting started sooner. Notice it also stains the glaze amber, it is not as clean as the frit. Notice the calculated thermal expansions: The greater melting of #2 and #3 comes at a cost, their thermal expansions are considerably higher, so they will be more likely to craze. Which of these is the best for functional ware? #1, G2926B (left). Its high SiO2 and enough-but-not-too-much B2O3 make it more durable. And it runs less during firing. And does not craze.
What material makes the tiny bubbles? The big bubbles?

This picture has its own page with more detail, click here to see it.
These are two 10 gram GBMF test balls of Worthington Clear glaze fired at cone 03 on terra cotta tiles (55 Gerstley Borate, 30 kaolin, 20 silica). On the left it contains raw kaolin, on the right calcined kaolin. The clouds of finer bubbles (on the left) are gone from the glaze on the right. That means the kaolin is generating them and the Gerstley Borate the larger bubbles. These are a bane of the terra cotta process. One secret of getting more transparent glazes is to fire to temperature and soak only long enough to even out the temperature, then drop 100F and soak there (I hold it half an hour).
Original glaze with Gerstley Borate vs. improved version with frit

This picture has its own page with more detail, click here to see it.
These pieces are fired at the same temperature. The glaze on the left is a popular recipe found online, Worthington Clear (our code number G2931). The Gerstley Borate in it is "farting" as the glaze is melting (the calculated LOI of the glaze as a whole is 15% mainly from that one material). Unless applied very thinly tons of micro-bubbles appear (this example is fired to cone 03). And strange, it is crazing badly also despite the low calculated thermal expansion. Using my account at insight-live.com I was able to source the B2O3 and MgO from a frit (actually two frits) to create the G2931K recipe. Although the thermal expansion calculates higher it is strangely fitting better (albeit not firing as white). As you can see, the new fritted glaze is very glassy and clear (thick or thin).
Close-up of Floating Blue on cone 6 dark/buff burning bodies

This picture has its own page with more detail, click here to see it.
This glaze recipe was originally popularized by James Chappell in the book "The Potter's Complete Book of Clay and Glazes", our code number is G2826R. It is and was loved and hated. Why? The high Gerstley Borate content makes it finicky. But the magic ingredient is not the GB, it is the rutile, Rutile makes the cobalt and iron dance. This recipe produces a number of different mechanisms of variegation. Color and opacity vary with thickness. Small rivulets of more fluid glass flow around more viscous phases producing micro-areas of differing colors and opacities. Titanium crystals sparkle and calcium-borate creates opalescence. Bubbles of escaping gases (from GB) have created pooling. Small black speckles from unground or agglomerated particles of iron are also present. Surprise! This is actually GR6-M Ravenscrag Floating blue. All the visuals, but without of the gelling and crazing headaches.
An example of how much Gerstley Borate LOI can affect a glaze

This picture has its own page with more detail, click here to see it.
Fired at cone 6. The samples on the bottom tiles are from ten-gram balls that have melted down (in our GBMF test). These glazes have the same chemistry, but the one of the left sources its B2O3 from Gerstley Borate (which has a high LOI). The one on the right gets it from a frit. Because the fritted version has less gases of decomposition to expel, the glass is much smoother. Curiously, the fritted version is flowing less and the red color has been lost. Why? This could be because the Al2O3, which stabilizes glazes against excessive fluidity, is being dissolved into the melt better and thus is more available for glass building.
Gerstley Borate vs Frit 3134 melt fluidity comparison

This picture has its own page with more detail, click here to see it.
Here the melt fluidity of Gerstley Borate (GB) is being compared to Ferro Frit 3134 (using a GLFL test). Clearly, these are two very different materials. GB is a clay, Frit 3134 is a man made powdered glass. Notice the GB shrinks to about half its original size by 1600F and then suddenly by 1650 it has exploded out of the starting gate and crossed the finish line! The frit, conversely, slowly softens through the entire 1350-1650 range and then starts down the runway between 1650 and 1700F. While it is clear that frit 3134 is not a direct substitute for the Gerstley Borate (GB) it's more gradual melting make it a better source as a source of B2O3 (boron).
Tried and True recipes. Really?

This picture has its own page with more detail, click here to see it.
Books and web pages with flashy pictures are the centrepiece of an addiction-ecosystem to recipes that often just don't work. Maybe these are "tried" by a lot of people. But are they "true"? Most are so-called "reactive glazes", outside normal practice - to produce visual interest they run, variegate, crystallize, pool, break, tint, go metallic, etc. But this happens at a cost. And inside special procedures and firing schedules that need explaining. It is not obvious these are understood by the recipe authors or sharers. And these recipes are dated and contain troublesome and unavailable materials. We use frits now to source boron. Stains are superior to raw colorants, even in glazes like this. Many of these will craze badly. And many will not suspend in the bucket. And will run during firing. Reactive glazes have other common issues: Blistering, leaching, cutlery marking, fuming. Trying colors in differing amounts in different base recipes is a good idea. But the project is most beneficial when it shows color response in terms of quality recipes of contrasting chemistries. The point of all of this: Understand a few glazes and develop them, rather than throwing spaghetti against the wall hoping something sticks. Commercial reactive glazes are an alternative also.
A frit softens over a wide temperature range

This picture has its own page with more detail, click here to see it.
Raw materials often have a specific melting temperature (or they melt quickly over a narrow temperature range). We can use the GLFL test to demonstrate the development of melt fluidity between a frit and a raw material. On the left we see five flows of boron Ferro Frit 3195, across 200 degrees F. Its melting pattern is slow and continuous: It starts flowing at 1550F (although it began to turn to a glass at 1500F) and is falling off the bottom of the runway by 1750F. The Gerstley Borate (GB), on the other hand, goes from no melting at 1600F to flowing off the bottom by 1625F! But GB has a complex melting pattern, there is more to its story. Notice the flow at 1625F is not transparent, that is because the Ulexite mineral within GB has melted but its Colemanite has not. Later, at 1700F, the Colemanite melts and the glass becomes transparent. Technicians call this melting behaviour "phase transition", that does not happen with the frit.
What to do about lithium carbonate and Gerstley Borate in glazes

This picture has its own page with more detail, click here to see it.
Lithium is getting really expensive. These are four recipes submitted by a customer who wonders if there is a substitute. The answer is not simple, each glaze is a unique situation. Fortunately, lithium carbonate is almost always a minor addition (in the first two recipes it is 1% and 3%). Lithium is a powerful low expansion flux, in some cases, a low melting low expansion frit could perform the same function (e.g. Ferro 3249). Even for the 6.5%, as in the third one, this could still work. But in these cases wouldn't it be better to continue using lithium? Even for the last one that has 9%? It's only expensive if you make glazes and don't use them. Perhaps a solution is to make them as brushing glazes, a 1-pint jar only needs about 350g of powder (that is only about 30g for recipe 4).
This situation can also be considered as an opportunity to rationalize the recipes you use. Let's pretend that each of these might be used on functional ware and should measure up to common sense recipe limits. The Gerstley Borate in three of these is also a red flag, that won't be available shortly (calculating how to source B2O3 from frits is better anyway). During that process, you might find that lithium is not even needed. Another issue is thermal expansion. Notice that one of these calculates to 8.5 and another to 9.6! Those are virtually certain to craze. Why not lower that number while removing the Gerstley Borate? Notice that two of these have clay percentages over 70% (Alberta Slip and Gerstley Borate), these are virtually certain to crack on drying (and crawl on firing), that can also be fixed. The percentage of titanium, Zircopax and rutile in the fourth one are guaranteed to make it crystallize heavily on cooling produce problems with cutlery marking and staining.
How does Gillespie Borate compare with the original Floating Blue recipe?

This picture has its own page with more detail, click here to see it.
The original Floating Blue recipe, our code number G2826R, has been popular for 50 years. But also troublesome (because of a fragile mechanism, poor slurry properties and inconsistencies in Gerstley Borate and rutile). Gillespie Borate, it's 2023 apparent successor, appears to solve most of its issues. These specimens of the recipe were fired using the cone 6 C6DHSC schedule. We have "vintage" Gerstley Borate from the 1990s, that is what was used here.
Top left: Floating Blue using Gerstley Borate (GB) (top) and Gillespie Borate bottom on a buff burning body.
Top right: Same but on a red burning body.
Centre: Melt fluidity GLFL test of the two glazes (GB) on the left.
Bottom: The two recipes and their calculated chemistries.
Clearly, the Floating Blue itself is firing greener than usual. And the Gillespie Borate version is much bluer. You may be used to something in between these two. The green tones could likely be restored by a reduction in the cobalt and increase in the iron oxide. The best news is that at 1.47 specific gravity, Gillespie Borate produces a far better slurry, there is no gelling. And no sign of settling into a hard layer.
The chemistry comparison at the bottom highlights some concerns, the difference is not insignificant. B2O3, Al2O3 and SiO2 are all lower (this could be part of the reason for the differences in color also). For better or worse, the melt fluidity is the same: Very high. This is likely because the percentage of Ulexite is higher (that melts better than Colemanite).
This glaze is not working with Gillespie Borate. What to do?

This picture has its own page with more detail, click here to see it.
These two mugs employ the same cone 6 pottery glaze recipe, the high-feldspar calcia matte (Ovo Perfect Matte). Like other mattes, it is high in calcium carbonate/wollastonite and kaolin but has no silica. But the one on the left has 13% Gerstley Borate while the one on the right uses Gillespie Borate. Gerstley Borate is a complex material, one that Mother Nature has uniquely endowed. It is a brown powder, a mix of two calcium borate minerals, ulexite and colemanite. And it is plastic, very plastic, from a hyper-fine particled clay (likely hectorite). And trace minerals. Gillespie Borate, by contrast, is a white powder, a synthetic blend attempting to replicate the obvious melting and physical properties of Gerstley Borate. It has, what some call, "a cleaner chemistry", enabling it to enhance rather than muddy whatever colorants are present. Any borate can melt well and foster crystallization, but Gerstley Borate is a mix of two borates that have different melting temperatures and patterns, this encourages phase separation and thus variegation in the aesthetic (its sub-micron clay particles may also act as catalysts).
What could be done? Add some iron to dirty up the material; if well dispersed in the slurry, 0.25% added to the recipe, might be sufficient. While Gillespie also has MgO, it might not be in the same form. A 1-2% addition of magnesium carbonate could help. And a small percentage of hectorite would provide some super-fine particles (with MgO).
Links
| URLs |
www.hamgil.com
Hamil & Gillespie web site |
| URLs |
https://www.lagunaclay.com/_files/ugd/e5330f_fdb75a4f2ff04a0a9bdb7aee308bd5a1.pdf
LagunaClay.com Gerstley Borate information page July 2011 A search for "Gerstley Borate" at the LagunaClay website produces no hits on Mar 2023. As of now, the price has jumped three-fold! |
| URLs |
https://insight-live.com/insight/share.php?z=HzyNzj9ELs
Replacing the Gerstley Borate in recipes containing 50% or more of it Many recipes were built on bases employing exceptionally high percentages of Gerstley Borate. At medium temperatures these melt fluid transparents hosted additions of colorants, variegators and opacifiers. At low fire they were used as transparents over underglaze decoration. |
| Materials |
Ulexite
A natural source of boron, it melts at a very low temperature to a clear glass. |
| Materials |
Colemanite
A natural source of boron that melts at a very low temperature. |
| Materials |
Boraq
This Gerstley Borate substitute was available during the early 2000s. Its recipe and development are well documented but two materials are no longer available. |
| Materials |
Gillespie Borate
A Gerstley Borate substitute that became available during the early 2000s and is still available in 2023. |
| Typecodes |
Flux Source
Materials that source Na2O, K2O, Li2O, CaO, MgO and other fluxes but are not feldspars or frits. Remember that materials can be flux sources but also perform many other roles. For example, talc is a flux in high temperature glazes, but a matting agent in low temperatures ones. It can also be a flux, a filler and an expansion increaser in bodies. |
| Typecodes |
Gerstley Borate Substitutes
Many development efforts to create Gerstley Borate substitutes took place during the early 2000s (the initial period when the demise of Gerstley Borate appeared imminent). A number of companies, including Laguna Clays itself, produced and sold these for many years. When Laguna secured another stockpile at the mine and began producing the original material again, interest in substitutes gradually waned. However, the sudden dramatic price increase in 2023 appears to have initiated the process again. Gillespie Borate appears to be the only viable and visible substitute now. Thus, the substitutes listed here are mostly no longer made. Other high-boron materials shown are also no longer available. We continue to recommend sourcing B2O3 from frits instead. Please contact us if you have a specific recipe and we can work with you in your Insight-live account to develop a new recipe that both eliminates the GB and improves overall working and firing properties. |
| Typecodes |
Gerstley Borate Glaze Calculation examples
Examples of how we use glaze calculations (in an Insight-Live.com account) to replace Gerstley Borate with other materials, especially frits, in various glaze recipes. In doing so we take the opportunity to improve the recipe in other ways (e.g. reduce thermal expansion, improve slurry properties, reduce bubbling and crawling). |
| Glossary |
Borate
Borate glazes, those fluxed with the oxide B2O3, are the most common type used in ceramic industry and hobby for low and medium temperatures. |
| Glossary |
Boron Blue
Boron blue is a glaze fault involving the crystallization of calcium borate. It can be solved using glaze chemistry. |
| Glossary |
Glaze Gelling
Glaze slurries can gel if they contain soluble materials that flocculate the suspension. Gelling is a real problem since it requires water additions that increase shrinkage. |
| Glossary |
Boron Frit
Most ceramic glazes contain B2O3 as the main melter. This oxide is supplied by great variety of frits, thousands of which are available around the world. |
| Glossary |
Trafficking
At Digitalfire we use the term "recipe trafficking" to describe the online trade in ceramic and pottery glaze recipes that can waste your time and cost you lots of money. Better to learn to understand glazes. |
| Oxides | B2O3 - Boric Oxide |
Video |
How I Developed the G2926B Cone 6 Transparent Base Glaze
How I found a pottery glaze recipe on Facebook, substituted a frit for the Gerstley Borate (using glaze chemistry), compared using a melt flow tester, added as much extra SiO2 as it would tolerate, and got a durable and easy-to-use cone 6 clear. |
| Media |
Getting Frustrated With a 55% Gerstley Borate Glaze
I show you why people love/hate this material and how I substituted it for Ulexite in this crazy recipe to make a far easier-to-use slurry that fires identical. |
| Media |
Thixotropy and How to Gel a Ceramic Glaze
I will show you why thixotropy is so important. Glazes that you have never been able to suspend or apply evenly will work beautifully. |
Data
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
