| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Suspension
In ceramics, glazes are slurries. They consist of water and undissolved powders kept in suspension by clay particles. You have much more control over the properties than you might think.
Key phrases linking here: suspension, suspending, suspended, settling, settled - Learn more
Details
In traditional ceramics, glazes are suspensions (or slurries), not solutions. They are mixes of insoluble mineral, frit and/or stain particles that have been added to water to form a liquid useful in the ceramic process. That suspension is what confronts us in the bucket or tank, learning how to assess and control it is critical to any ceramic process.
The particles normally stay suspended in the slurries because of the presence of clay particles (e.g. kaolin, ball clay, bentonite or other clay like mineral whose particles have an electrolytic affinity for water). Clay particles typically form a 'house-of-cards' structure within the suspension because the particles are flat and have opposing electrolytic charges on their faces and edges. This structure holds other particles in suspension. The specific gravity, the types of the clay(s) used to suspend, the pH of the water and the nature of the interactions of particles of all materials present (according to their shapes, size, surface topologies, electrolytic character) interplay to determine the gelling, thixotropic and other rheological properties of the slurry. Organic binders and/or flocculants/deflocculants are often added to modify the natural suspension characteristics of the mix, the amount of water needed, the degree of dry hardness and the gel and viscosity behaviour.
Glazes used in large-scale manufacturing must have a high specific gravity (e.g. 1.7) so they can go on thick in one coat and dry quickly with minimal shrinkage (because ware is not bisque fired). Most potters would find these impossible to use but industry has developed machinery that works with them. Those used in traditional pottery and functional applications, where the ware has been bisque fired, can and should have a higher water content (a specific gravity as low as 1.4). This enables adding an agent to gel the slurry for easier and more even application, stays more stable over time and is easily adjusted if needed.
Glaze recipes for use in pottery almost always appear with no instructions about how much water to use. This is not entirely inappropriate since one of the variables is electrolyte levels in water, your water could flocculate or deflocculate it. Your working preferences are another variable. It is thus appropriate to develop or discover your best mixing procedure for the recipe. In addition, many recipes have anomalies that make them inherently difficult to use. Thus it is entirely appropriate to make certain modifications immediately. This is especially the case with clay content. Clays of course include kaolin, ball clay, other plastic clays, but also Gerstley Borate (it is highly plastic). Glazes for traditional ceramics work best with 15-20% kaolin (most common) or ball clay. Although you might not think that is enough, keep reading, you will see why. Certain kaolins and ball clays create better glaze slurries than others, substitute the one you have found works best (kaolin chemistries are very similar). If a glaze has 20% or more clay content then no bentonite is needed (remove it if present, you will see why below). If clay content exceeds 25% then shrinkage could be enough that the glaze will crack on drying. The solution is to use a mix of raw and calcined clay. Glazes having raw borate (like Gerstley Borate) often also contain significant kaolin, the combined amount can easily exceed 50%. They definitely do not need bentonite, rather, they need a reduction in plastic clay to reduce drying shrinkage. Substitute some or all of the kaolin with calcined kaolin (using 12% less, see the calcination page for why this is).
Sometimes settling properties of glazes can change dramatically by the addition of a material that would seem unrelated (e.g. a stain), in these cases that specific variation of the recipe must be treated as a special case to optimize its suspension properties.
The field of glaze rheology (suspension properties) is one of the most important applications of glaze chemistry. Why? Because it is possible to maintain the chemistry (which determines fired properties) while adjusting the mix of materials that source the oxides to produce that chemistry. For example, Al2O3 can be sourced from a frit, a feldspar or a clay (or a different clay than the one in the recipe). The one you choose depends on which is better for the working and suspension properties needed. Optimizing the suspension properties of a glaze can have a dramatic impact on its ease-of-use, and in turn, the quality of the fired ware.
Most technicians, especially potters, do not realize that almost any glaze recipe, provided it does not contain too much raw clay, can be suspended and made into a beautiful-to-use slurry that applies evenly without drips. Even if it has almost no clay! How? It is not necessary to use an ancient Japanese concoction of boiled seaweed mixture! Just add a flocculant to gel the slurry (see the thixotropy page referenced below). Even better, do that in combination with an addition of bentonite or Veegum.
Related Information
The ball clay you use to suspend your glaze is important!
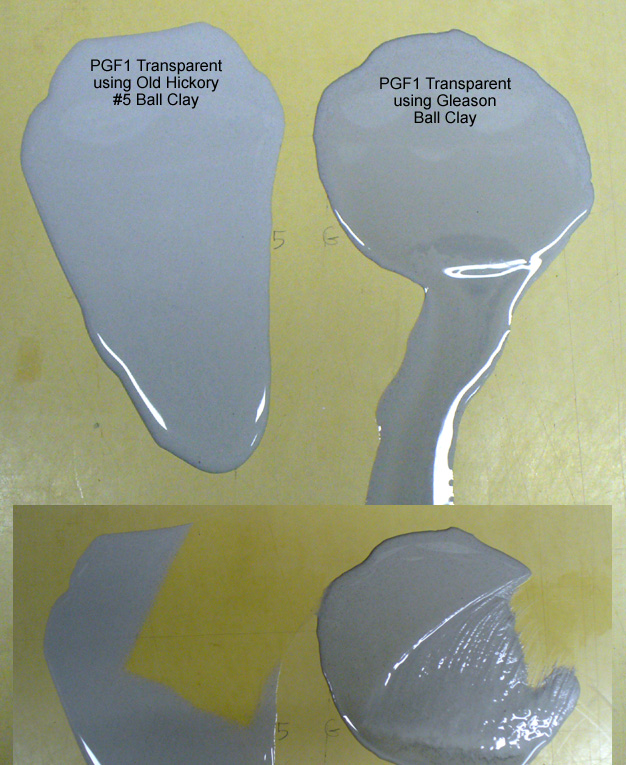
This picture has its own page with more detail, click here to see it.
I poured 4 teaspoons of two glazes onto a non-absorbent butcher’s board and let them sit for a minute, then inclined the board. The one on the right employs Gleason Ball clay, the left one has Old Hickory #5 ball clay. Neither has any rheology modifier additions. The one on the right has settled and, on incline of the board, the watery upper is running off. The other has gelled and the whole thing is running downward slowly. Below that you can see where I have begun to sponge them off, the one on the right is sticky. The most amazing thing about this: This difference appears despite the fact that there is only 7% ball clay in this heavily fritted recipe.
Yikes, the glaze on the right is going on way too thick and drying too fast

This picture has its own page with more detail, click here to see it.
Sometimes EP Kaolin is the best suspender in a glaze, sometimes it isn't. These are the same 85% fritted glaze. A (left) employs 15% Old Hickory #5 ball clay to suspend it, B (right) has 15% EPK. B settles quickly, demands low water content or it runs like water, it goes on very thick even if dipped quickly, it dries instantly and creates uneven thicknesses. By contrast, A goes on like silk, doesn't settle, dries evenly in about 10 seconds. What a difference! All simply because of using a different clay to suspend it.
Fundamentals of Fluid Mechanics - book

This picture has its own page with more detail, click here to see it.
Many aspects of ceramic production relate to the control of fluids (mostly suspensions). This is also true of material production. If you want to solve problems and optimize your process this is invaluable knowledge. This book is available at amazon.com.
Suspending pure feldspar and applying it as a glaze

This picture has its own page with more detail, click here to see it.
Most people think that would be impossible. But it is not. This slurry will stay in suspension for days. How? It is flocculated using a tiny bit of powdered epsom salts. Without the epsom salts it is watery and will settle in seconds. How does the slurry apply to this porcelain? Since it contains no clay it has complete permeability. Against the immersed bisque a layer builds very rapidly, pieces must be dipped and removed immediately. Does it dry hard enough to handle? Yes.
A pure silica suspension that behaves like a glaze. Is that possible? Yes.

This picture has its own page with more detail, click here to see it.
This slurry is just water and 295 mesh silica. I have mixed it to 1.79 specific gravity and it is creamy. It applies like a glaze to bisque ware (if I dip it fast) and goes on super smooth and even. It does settle, but only slowly. Unlike feldspar and nepheline syenite, if I thin it a little and add powdered epsom salts or vinegar it does not gel, no matter how much I put in. The only response I can see is that it appears to settle out a little less. I was always taught that clay is needed to suspend things, every thing else will settle out like a rock if there is no clay present in the slurry. Of any material, this is one that I would have expected to settle out the fastest.
Two glazes. One crawls, the other does not. Why?

This picture has its own page with more detail, click here to see it.
The glaze on the right is crawling at the inside corner. Why? Multiple factors contribute. The angle between the wall and base is sharper. A thicker layer of glaze has collected there (the thicker it is the more power it has to impose a crack as it shrinks during drying). It also shrinks more during drying because it has a higher water content. But the leading cause: Its high raw clay content increases drying shrinkage. Calcining part of the raw clay destroys its affinity for water (which is what makes it plastic), this is an effective way to deal with this. Or doing a little chemistry to source some of the Al2O3 from materials other than clay (e.g. a frit having a higher Al2O3 content).
Links
| Glossary |
Ceramic Slip
The term Slip can have various meanings in traditional ceramics. |
| Glossary |
Water in Ceramics
Water is the most important ceramic material, it is present every body, glaze or engobe and either the enabler or a participant in almost every ceramic process and phenomena. |
| Glossary |
Thixotropy
Thixotropy is a property of ceramic slurries of high water content. Thixotropic suspensions flow when moving but gel after sitting (for a few moments more depending on application). This phenomenon is helpful in getting even, drip-free glaze coverage. |
| Glossary |
Calcination
Calcining is simply firing a ceramic material to create a powder of new physical properties. Often it is done to kill the plasticity or burn away the hydrates, carbonates, sulfates of a clay or refractory material. |
| Glossary |
Slurry Processing
|
| Troubles |
Crawling
Ask yourself the right questions to figure out the real cause of a glaze crawling issue. Deal with the problem, not the symptoms. |
| Troubles |
Foaming of Ceramicd Glaze Slurries
Ceramic glaze slurries can sometimes generate enough foam that it becomes difficult to apply an even layer to a surface. What can you do? |
| Troubles |
Powdering, Cracking and Settling Glazes
Powdering and dusting glazes are difficult and a dust hazard. Shrinking and cracking glazes fall off and crawl. The cause is the wrong amount or type of clay. |
| Media |
A Broken Glaze Meets Insight-Live and a Magic Material
Use Insight-Live.com to do major surgery on a feldspar saturated cone 10R glaze recipe with multiple issues: blistering, pinholing, crazing, settling, dusting and possibly leaching! |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
