| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Kaolin
Alternate Names: China Clay, Generic Kaolin
Description: Hydrated alumina silicate, Pure clay mineral
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| Al2O3 | 40.21% | 1.00 | |
| SiO2 | 47.29% | 2.00 | |
| LOI | 12.50% | n/a | |
| Oxide Weight | 221.96 | ||
| Formula Weight | 253.67 | ||
Notes
A wide array of kaolin (also known as China Clay) products are available. These vary in plasticity, crystal and surface chemistry, particle shape and size, flow properties, permeability, etc. However the most common varieties most people will see are two: kaolins intended for plastic bodies or casting ones. Plastic kaolins can rival the workability of a ball clay, casting ones can be so short that it is difficult to even wedge or roll them without the plastic mass falling apart. Strangely, non-plastic kaolins are not necessarily whiter burning.
Pure kaolin is the clay of choice for bodies that need to be clean and white. Many porcelains contain only a kaolin mix as their clay complement. But kaolins have relatively low plasticity when compared to other raw clay types. Thus in non-casting plastic-forming bodies it is often not possible to achieve enough plasticity employing kaolin alone. Additions of ball clays, bentonites and other plasticizers are thus common. Where translucency and whiteness are paramount, highly plastic kaolins and white burning ball clays and bentonites can be used .
Because kaolinite mineral has a much larger particle size than ball clay and bentonite materials, blending it with them in bodies can produce a good cross-section of ultimate particle sizes (this imparts enhanced working and drying properties). Another advantage of the larger particle size of kaolins is that they are much more permeable to the passage of water. Thus kaolins, especially the larger sized ones, speed up casting rates in slurry bodies and drying rates in all bodies.
Kaolins are employed in glaze recipes to keep the silica, feldspar, frit and other particles from settling out (the surface chemistry of the particles and their interaction with water are responsible for this behavior). At the same time the oxide chemistry of kaolin makes it the primary source of alumina oxide for glazes.
Kaolin is a very refractory aluminum silicate. Kaolin-based bodies are used to make all kinds of refractory parts for industry. Kiln wash is often made from 50:50 mix of kaolin and silica. Cordierite is made mainly from kaolin. High-heat-duty grogs are made by calcining kaolin.
Kaolin is used in many industries other than ceramics, in fact the ceramics industry uses only a small amount of the total kaolin produced. Kaolin companies tend to be billion-dollar operations and kaolin is used in everything from paper to cosmetics, paint to agricultural products. The spread of pictures across the page at the Ukranian Kaolin Company shows some examples: http://www.ukc-kaolin.com/en/product.html
If you use kaolin in your production there is good reason to be doing routine quality control to make sure it remains consistent. Kaolins can sometimes have particulate impurities (can cause firing specks) and exhibit differences in soluble salts content, drying shrinkage, drying performance and behavior in slurries. Different name-brand kaolins, and even different ones marketed by the same company, can have very different properties. The plasticity range can be huge (from almost zero to like ball clay). Fired color and fired maturity can be very different. If you make a porcelain, for example, it is very important to compare fired maturity of the recipe using the original and new kaolin (using the SHAB test for example). It is often necessary to compensate fired maturity changes by adjusting the feldspar percentage. And plasticity changes by adding/removing bentonite or adjusting the ball clay/kaolin proportion. Also, just because a clay fires really white does not mean it is a koalin, even if the supplier calls it such.
Kaolin transforms to mullite above 1000C, this is a key factor in the micro structure of porcelain and other types of bodies. This transformation is also exploited in engobes.
Related Information

This picture has its own page with more detail, click here to see it.
Ball clay and kaolin test bars side-by-side fired from cone 9-11 oxidation and 10 reduction.
How a kaolin and ball clay compare in a dry performance test
This picture has its own page with more detail, click here to see it.
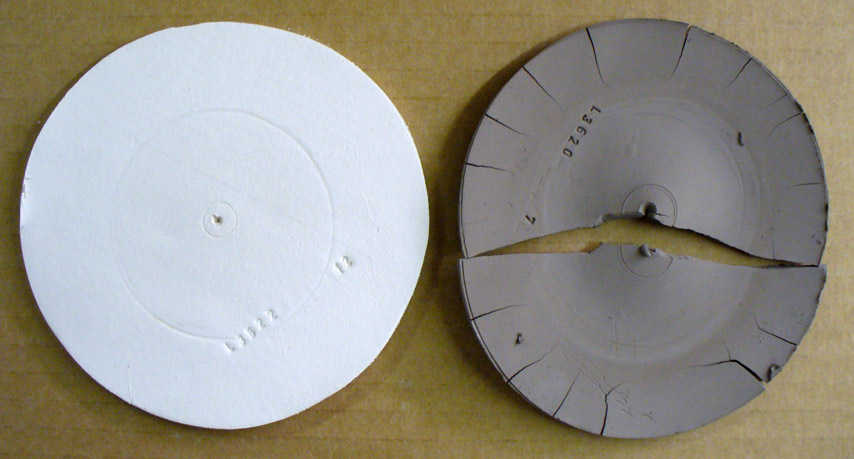
These are DFAC drying performance disks of a large-particle kaolin (OptiKast) and a ball clay (Plainsman A2). This test reveals a clay's response to uneven drying (these disks are dried with the center portion covered). The kaolin feels smoother yet its ultimate particles are ten to one hundred times bigger than a typical ball clay. Thus it shrinks much less. The ball clay has dramatically lower water permeability, water from the center protected portion resists migration to the outer edge during drying. When the inner section finally dried the outer was already rigid so it split the disk in two and pulled all the edge cracks. Most ball clays shrink more and crack worse than this (cracks concentric to the center also appear). So why use ball clay? This kaolin is so lacking in plasticity it was barely possible to even make this disk. And it is so weak that it can easily break just by handling it. Still, it is useful to make casting bodies. But the ball clay, when used as a percentage of a body mix, can produce highly plastic bodies than can be dried without trouble if done evenly.
Closeup of Halloysite particles

This picture has its own page with more detail, click here to see it.
Electron micrograph showing Dragonite Halloysite needle structure. For use in making porcelains, Halloysite has physical properties similar to a kaolin. However it tends to be less plastic, so bodies employing it need more bentonite or other plasticizer added. Compared to a typical kaolin it also has a higher fired shrinkage due to the nature of the way its particles densify during firing. However, Dragonite and New Zealand Halloysites have proven to be the whitest firing materials available, they make excellent porcelains.
Does this terra cotta clay have an LOI higher than kaolin? No.

This picture has its own page with more detail, click here to see it.
These two samples demonstrate how different the LOI can be between different clay minerals. The top one is mainly Redart (with a little bentonite and frit), it loses only 4% of its weight when fired to cone 02. The bottom one is New Zealand kaolin, it loses 14% when fired to the same temperature! The top one is vitrified, the bottom one will not vitrify for another 15 cones.
Health warning phrases on a bag of Kaolin

This picture has its own page with more detail, click here to see it.
880 bags of kaolin arrive. First step: Record the date code.

This picture has its own page with more detail, click here to see it.
A shipment EP Kaolin has arrived for use in some of our production porcelain and stoneware bodies. Of course, this needs to be tested before being put into product. But how? The first step is to create a new recipe record in my Insight-Live account, and find their production date code stamp on the bag. Hmmm. It does not have one! OK, then I need to record the date on which we received it. We need to save a bag on every pallet and sieve 50 grams through 100 mesh (to spot contamination). Then we'll make test bars (of all the samples mixed) to fire across a range of temperatures (to compare fired maturity with past shipments). We do a drying performance disk also to assess soluble salts.
Cone 6 kaolin porcelain verses ball clay porcelain.

This picture has its own page with more detail, click here to see it.
Typical porcelains are made using clay (for workability), feldspar (for fired maturity) and silica (for structural integrity and glaze fit). These cone 6 test bars demonstrate the fired color difference between using kaolin (top) and ball clay (bottom). The top one employs #6 Tile super plastic kaolin, but even with this it still needs a 3% bentonite addition for plasticity. The bottom one uses Old Hickory #5 and M23, these are very clean ball clays but still nowhere near the whiteness of kaolins. Plus, 1% bentonite was still needed to get adequate plasticity for throwing. Which is better? For workability and drying, the bottom one is much better. For fired appearance, the top one.
Ball clay vs. Kaolin porcelain at cone 6

This picture has its own page with more detail, click here to see it.
Left: A porcelain that is plasticized using only ball clays (Spinx Gleason and Old Hickory #5). Right: Only kaolin (in this case Grolleg). Kaolins are much less plastic so bentonite (e.g. 2-5%) is typically needed to get good plasticity. The color can be alot whiter using a clean kaolin, but there are down sides. Kaolins have double the LOI of ball clays, so there are more gasses that potentially need to bubble up through the glaze (ball clay porcelains can produce brilliantly glassy and clean results in transparent glazes even at fast fire, while pure kaolins can produce tiny dimples in the glaze surface if firings are not soaked long enough). Kaolins plasticized by bentonite often do not dry as well as ball clays even though the drying shrinkage is usually less. Strangely, even though ball clays are so much harder and stronger in the dry state, a porcelain made using only ball clays often still needs some bentonite. If you do not need the very whitest result, it seems that a hibrid using both is still the best general purpose, low cost answer.
Do not rely on material data sheets, do the testing

This picture has its own page with more detail, click here to see it.
The cone 6 porcelain on the left uses Grolleg kaolin, the right uses Tile #6 kaolin. The Grolleg body needs 5-10% less feldspar to vitrify it to zero porosity. It thus contains more kaolin, yet it fires significantly whiter. Theoretically this seems simple. Tile #6 contains alot more iron than Grolleg. Wrong! According to the data sheets, Grolleg has the more iron of the two. Why does it always fire whiter? I actually do not know. But the point is, do not rely totally on numbers on data sheets, do the testing yourself.
What happens when you dry and bisque a piece made of pure kaolin?

This picture has its own page with more detail, click here to see it.
The way in which the walls of this bisque fired kaolin cup laminate reflect the plately and uniform nature of the kaolin particles. Because they are lining up during the wedging and throwing process, the strength to resist cracks is better along the circumference than perpendicular to it. The bonds are weak enough that it is very easy to break it apart by hand (even though it is bisque fired). The worst laminations were at the bottom where wall thickness was the most variable and therefore the most drying stresses occurred. However, if this kaolin were blended with feldspar and silica, this lamination tendency would completely disappear.
Cleanest kaolin porcelain vs. ball-clay-only porcelain!

This picture has its own page with more detail, click here to see it.
These cone 6 clear-glazed porcelains demonstrate just how white you can make a porcelain if you use white burning kaolins and bentonites instead of ball clays. Both contain about 40% clay. The one on the left employs New Zealand kaolin and Veegum plasticizer, the one on the right Kentucky ball clays (among the whitest of ball clays in North America) and standard bentonite. Both are zero porosity. The glaze surface is a little more flawless on the right one (possibly because ball clays have a lower LOI than kaolins).
Chunks of metal found in contaminated truckload of kaolin

This picture has its own page with more detail, click here to see it.
You may not fully appreciate what your clay body manufacturer has to go through to make clean porcelain for you. Every load of material that they receive has to be checked. We now have to check every pallet. This is the third semi-trailer load of material we have had contaminated (ball clays and kaolins are most vulnerable). When we phoned another manufacturer they checked their supply and it was contaminated also! Materials can also be contaminated by larger clay particles that disrupt the fired glaze surface. These chunks of metal were pulled out by magnets in the production line, a thousand boxes of porcelain are now garbage. It is too expensive to return a load, so it just becomes a loss.
What material makes the tiny bubbles? The big bubbles?

This picture has its own page with more detail, click here to see it.
These are two 10 gram GBMF test balls of Worthington Clear glaze fired at cone 03 on terra cotta tiles (55 Gerstley Borate, 30 kaolin, 20 silica). On the left it contains raw kaolin, on the right calcined kaolin. The clouds of finer bubbles (on the left) are gone from the glaze on the right. That means the kaolin is generating them and the Gerstley Borate the larger bubbles. These are a bane of the terra cotta process. One secret of getting more transparent glazes is to fire to temperature and soak only long enough to even out the temperature, then drop 100F and soak there (I hold it half an hour).
Bubbles in Terra Cotta transparent glazes. What to do?

This picture has its own page with more detail, click here to see it.
Two transparent glazes applied thickly and fired to cone 03 on a terra cotta body. Right: A commercial bottled clear, I had to paint it on in layers, I ended up getting it on pretty thick. Left: G1916S, a mix of Ferro frits, nepheline syenite and kaolin - one dip for 2 seconds and it was glazed. And it went on more evenly. Bubbles are, of course, generated by and clay body during firing, but terra cottas are the worst. And when fired toward vitrification the gas volume can really increase. Complicating this is the fact that low temperature glazes melt early, while body gassing may still be happening. Improvements? Both of these could have been applied thinner. And I could have fired them using a drop-and-hold and a slow-cool schedule. But the biggest improvement would likely be firing lower, to cone 04.
The difference in fired character between kaolin and ball clay at cone 10R

This picture has its own page with more detail, click here to see it.
The top one is EP Kaolin, the bottom one is Old Hickory M23 Ball Clay (these materials are typical of their respective types). These materials have low alkali contents (especially the kaolin), this lack of flux means they are theoretically highly refractory mixes of SiO2 and Al2O3. It is interesting that, although the kaolin has a much larger ultimate particle size, it is shrinking much more (23% total vs. 14%). This is even more unexpected since, given that it has a lower drying shrinkage, and should be more refractory. Further, the kaolin has a porosity of 0.5% vs. the ball clay's 1.5%. The kaolin should theoretically have a much higher porosity? What is more, both of these values are unexpectedly low. This can partly be explained by the particle packing achieved because of the fine particle size. Despite these observations, their refractory nature is ultimately proven by the fact that both of these can be fired much higher and they will only slowly densify toward zero porosity.
We have to fight with the fibreglass industry to get kaolin!

This picture has its own page with more detail, click here to see it.
These are bags from three recent truckloads of 880 bags each. Order-delivery delays are getting longer and longer as the fibreglass industry is making more and more demands on kaolin suppliers. This means we have to store this material in larger quantities and for longer periods than in the past. And we must be more diligent in testing for consistency because manufacturers are catering to fibreglass instead of ceramics. When this is coupled with the decline of ceramic manufacturing in North America it means maintaining and documenting the properties important to ceramics are becoming less important to kaolin manufacturers.
Two kaolins, one cracks on bisque, the other does not

This picture has its own page with more detail, click here to see it.
Both of these are mixed 70:30 kaolin:feldspar. Left is a fine particled kaolin, #6 Tile. Right, a coarser particled, less plastic material, EPK. During forming, the larger particles of the latter line up concentrically to the center better. This causes the body to shrink more along radius lines than along tangents, producing these cracks (many were made and they all cracked like this). In addition, bodies employing kaolin as the only clay in the recipe often fail like this because they lack the “stress-relief plasticity” that a bentonite or ball clay addition provides. The Tile#6 material on the left contains natural bentonite, so it can better relieve drying stresses.
How plastic is a pure kaolin? Could one use it pure for pottery?

This picture has its own page with more detail, click here to see it.
Pure kaolins are clay. It seems logical that "pure clay" is plastic. However most kaolins are not plastic (compared to a typical clay for throwing or modelling). This is because they have a comparatively large particle size (compared to ball clays, bentonites, etc). This small bowl was thrown from #6 Tile kaolin. It is, by far, the most plastic kaolin available in North America. It's throwing properties are so good that one might be misled into thinking it should be possible to make pottery from it. Unfortunately, if it was survive drying without cracks, it would not make it through firing without this happening. This was fired, unglazed, to cone 6. Pure kaolin particles are flat and the throwing process lines them up concentric to centre. So shrinkage is greater across than along them. A filler is needed to separate the kaolin particles. All pure kaolins are also refractory, so even if this bowl had not cracked, the porosity of this piece is very high, completely impractical for functional ware (it needs a flux like feldspar to develop fired maturity).
Will soil testing help you assess a wild clay for pottery?

This picture has its own page with more detail, click here to see it.
No, soil testing is not helpful. Soils normally contain clay but it is so diluted with sand, rocks, silt and organics that overall plasticity is just a dot on a graph - not even close to what modelling or throwing clays exhibit. Pottery clays easily hold a shape and can be adjusted to a new shape without splitting. They dry slowly with substantial shrinkage. Highly plastic clays need more water to achieve working consistency, silty non-plastic ones need less (typical pottery clays need 18-23%). The reports shown here are typical for soils. But almost nothing here would look familiar to a potter.
-The Shrinkage Limit (SL) is the water content where further loss of moisture will not result in any more volume reduction.
-The Plastic Limit (PL) is minimum water content at which a soil is considered to behave in a ‘plastic’ manner, i.e. is capable of being moulded.
-The Liquid Limit (LL) is the maximum water content a silt or clay can have before becoming a liquid, i.e. turning into mud.
-The Plasticity Index (PI) is the range of moisture contents where the silt or clay remains plastic (PI = LL – PL).
Potters don't care about the amount of water needed, they care about how plastic the clay is once enough water has been added to get the right stiffness.
Links
| Materials |
Ajax P Kaolin
|
| Materials |
Allen G Kaolin
|
| Materials |
Glomax LL Kaolin
|
| Materials |
A Kaolin
|
| Materials |
600 Kaolin
|
| Materials |
Albion Sperse
|
| Materials |
Avery Kaolin
|
| Materials |
B Kaolin
|
| Materials |
Bell Kaolin
|
| Materials |
Puraflo 50 Kaolin
|
| Materials |
C Kaolin
|
| Materials |
Calcined Kaolin
This is kaolin powder that has been fired in a furnace to remove the 12% crystal water and render it non-plastic. |
| Materials |
CC China Clay
|
| Materials |
CC31 China Clay
|
| Materials |
Ceraclay WTC Kaolin
|
| Materials |
WTD Kaolin
|
| Materials |
CF Kaolin
|
| Materials |
D Kaolin
|
| Materials |
Diamond Kaolin
|
| Materials |
E Kaolin
|
| Materials |
English Kaolin
|
| Materials |
EP Kaolin
A kaolin that gels slurries (thus handy to suspend ceramic glazes). It is plastic and fires white enough that it is also valuable in porcelain bodies. |
| Materials |
Ewing Kaolin
|
| Materials |
F. C. Kaolin
|
| Materials |
Glomax LL Calcined Kaolin
|
| Materials |
Grolleg Kaolin
A white burning kaolin from the UK, commonly used in porcelain bodies and as a glaze suspender. Sticky when wet, low plasticity. |
| Materials |
Gunheath Kaolin
|
| Materials |
Hamilton Kaolin
|
| Materials |
Helmer Kaolin
|
| Materials |
Hillman Kaolin
|
| Materials |
Hong Kong Kaolin
|
| Materials |
IXL 34E
|
| Materials |
IXL 34E/F 95
|
| Materials |
IXL RE
|
| Materials |
JHC White Kaolin
|
| Materials |
K1 Kaolin
|
| Materials |
K50 Kaolin
|
| Materials |
Kamec Kaolin
|
| Materials |
Kampaku Kaolin
|
| Materials |
Kaolin Slurry
|
| Materials |
Kaopaque 20 Kaolin
|
| Materials |
Kingsley Kaolin
|
| Materials |
KMS Kaolin
|
| Materials |
Korean Kaolin
|
| Materials |
KT-Cast Kaolin
|
| Materials |
LG Kaolin
|
| Materials |
LPC Kaolin
|
| Materials |
Mastercast Kaolin
|
| Materials |
Masterfil Kaolin
|
| Materials |
Masterfloat Kaolin
|
| Materials |
McNamee Kaolin
|
| Materials |
Monarch Kaolin
|
| Materials |
No. 50 China Clay
|
| Materials |
NSC Kaolin
|
| Materials |
Peerless 2 Kaolin
|
| Materials |
Pioneer Kaolin
|
| Materials |
Piopot Kaolin
|
| Materials |
Pleyber S Kaolin
|
| Materials |
Putnam Kaolin
|
| Materials |
Putnam S Kaolin
|
| Materials |
Qc China Clay
|
| Materials |
Rampant BB Kaolin
|
| Materials |
Remblend Kaolin
|
| Materials |
Rogers Kaolin
|
| Materials |
SA-1 Kaolin
|
| Materials |
SAF Kaolin
|
| Materials |
Samson Kaolin
|
| Materials |
Sapphire Kaolin
|
| Materials |
Snobrite Kaolin
|
| Materials |
Standard Porcelain Kaolin
|
| Materials |
Stannon Kaolin
|
| Materials |
Tile #6 Kaolin
|
| Materials |
Treviscoe Kaolin
|
| Materials |
Plainsman Troy Clay
|
| Materials |
Velvacast Kaolin
|
| Materials |
WC-5 Kaolin
|
| Materials |
Wilclay CR Kaolin
|
| Materials |
Wilclay WC Kaolin
|
| Materials |
Wilson Kaolin
|
| Materials |
WS Kaolin
|
| Materials |
KAOLIN-SERPENTINE
|
| Materials |
Lampang Kaolin
|
| Materials |
Zedlec Kaolin
|
| Materials |
Delta Kaolin
|
| Materials |
Kaolex D-6 Kaolin
|
| Materials |
Laguna #1 Kaolin
|
| Materials |
Snocal 707 Kaolin
|
| Materials |
Grade C Kaolin
|
| Materials |
Sovereign Kaolin
|
| Materials |
Kaolin Natural BP
|
| Materials |
Speswhite Kaolin
|
| Materials |
Super Standard Porcelain
|
| Materials |
Stockalite Kaolin
|
| Materials |
Supreme Kaolin
|
| Materials |
Topaz Kaolin
|
| Materials |
DB Float Kaolin
|
| Materials |
Allen Kaolin
|
| Materials |
James Bay Stn Kaolin
|
| Materials |
JB Kaolin
|
| Materials |
Kaolin 111
|
| Materials |
Kaolin 113
|
| Materials |
Kaolin 114
|
| Materials |
Kaolin 115
|
| Materials |
Kaolin 143
|
| Materials |
Kaolin 151
|
| Materials |
Kaolin 171
|
| Materials |
D'Arvor Kaolin
|
| Materials |
H-1 Kaolin
|
| Materials |
Sparks Kaolin
|
| Materials |
609 Kaolin
|
| Materials |
Layton Clay
|
| Materials |
1431 Kaolin
|
| Materials |
1433 Kaolin
|
| Materials |
1434 Kaolin
|
| Materials |
1479 Kaolin
|
| Materials |
38 Kaolin
|
| Materials |
Klondike Kaolin
|
| Materials |
VC-1 Kaolin
|
| Materials |
A-1 Kaolin
|
| Materials |
Richardsons Kaolin
|
| Materials |
M+M China Kaolin
|
| Materials |
M.G.R. Kaolin
|
| Materials |
M.W.M. Kaolin
|
| Materials |
No. 44 Kaolin
|
| Materials |
No. 17 Kaolin
|
| Materials |
372 Kaolin
|
| Materials |
Dixie Clay
|
| Materials |
Langford Kaolin
|
| Materials |
Bilt-Cote Kaolin
|
| Materials |
PAR Kaolin
|
| Materials |
Snocal 40
|
| Materials |
Peerless 3 Kaolin
|
| Materials |
Sterling Kaolin
|
| Materials |
Kernick Kaolin
|
| Materials |
Ball Clay
A fine particled highly plastic secondary clay used mainly to impart plasticity to clay and porcelain bodies and to suspend glaze, slips and engobe slurries. |
| Materials |
Zettlitzer Kaolin
|
| Materials |
Caolín Gris (Cuba)
|
| Materials |
Caolín Blanco (Cuba)
|
| Materials |
Caolín Mantua (Cuba)
|
| Materials |
Caolín Damanueco (Cuba)
|
| Materials |
Caolín Santa Elena (Cuba)
|
| Materials |
Eckaglass Kaolin
|
| Materials |
Wilco-UPF Kaolin
|
| Materials |
Glasurfritten 33.17
|
| Materials |
SKT-1433 Kaolin
|
| Materials |
Spinks Kaolin Low Residue
|
| Hazards |
Clay Toxicity
Is clay toxic? No. Should you breathe it? No. |
| Typecodes |
Kaolin
Pure clay mineral, there are many brand names of varying purity and iron content. |
| Typecodes |
Generic Material
Generic materials are those with no brand name. Normally they are theoretical, the chemistry portrays what a specimen would be if it had no contamination. Generic materials are helpful in educational situations where students need to study material theory (later they graduate to dealing with real world materials). They are also helpful where the chemistry of an actual material is not known. Often the accuracy of calculations is sufficient using generic materials. |
| Typecodes |
Kaolin
Pure clay mineral, there are many brand names of varying purity and iron content. |
| URLs |
http://kaolin.com/
Information at Kaolin.com |
| URLs |
http://en.wikipedia.org/wiki/Kaolin
Kaolin at Wikipedia.com |
| Oxides | Al2O3 - Aluminum Oxide, Alumina |
| Oxides | SiO2 - Silicon Dioxide, Silica |
| Minerals |
Kaolinite
The most fundamental clay mineral. This mineral is found in nature in its purest form as kaolin. How |
| Glossary |
Clay
What is clay? How is it different than dirt? For ceramics, the answer lies on the microscopic level with the particle shape, size and how the surfaces interact with water. |
| Glossary |
Plasticity
Plasticity (in ceramics) is a property exhibited by soft clay. Force exerted effects a change in shape and the clay exhibits no tendency to return to the old shape. Elasticity is the opposite. |
| Glossary |
Porcelain
How do you make porcelain? There is a surprisingly simple logic to formulating them and to adjusting their working, drying, glazing and firing properties for different purposes. |
| Glossary |
Permeability
In ceramics, the permeability of clay slurries and plastics determines the rate as which water can move through the matrix |
| Projects |
Materials
|
| Projects |
Troubles
|
| Tests |
Shrinkage/Absorption Test
SHAB Shrinkage and absorption test procedure for plastic clay bodies and materials |
| Tests |
Sieve Analysis 35-325 Wet
A measure of particle size distribution by washing a powdered or slaked sample through a series of successively finer sieves |
| Articles |
Formulating a Porcelain
The principles behind formulating a porcelain are quite simple. You just need to know the purpose of each material, a starting recipe and a testing regimen. |
Data
| Frit Softening Point | 1770C M |
|---|---|
| Density (Specific Gravity) | 2.62 |
Mechanisms
| Body Plasticity | It is possible to make a plastic throwing body using 50% kaolin only, however you must choose one of the highly plastic varieties such as #6 Tile. Even then you will likely need a little bentonite to augment the kaolin. There is a huge range in kaolin plasticities, test for yourself to find out. |
|---|---|
| Glaze Suspender | Kaolin is the most common glaze suspender. Depending on the type of kaolin used, 15-20% should be enough. Many fritted glazes are composed solely of frit and kaolin. Some kaolins make the glaze gel, this is a helpful additional mechanism to keep it suspended. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
