| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
B2O3 (Boric Oxide)
Data
| Co-efficient of Linear Expansion | 0.031 |
|---|
Notes
-B2O3 is a low melting glass of low thermal expansion and surface tension. It is an extremely useful oxide, indispensable in many industries and applications. Na2O melts as well or better than B2O3, but unlike B2O3, its high thermal expansion limits it to lower percentages.
-Below cone 10, additions of B2O3 are almost always needed to make glazes melt well. Other fluxes, like ZnO, will melt glazes at cone 6, but many issues limit them to only certain types of glazes. The lower the temperature the more boron is needed. A melt fluidity checker is the best way to determine if the glaze is melting enough (and not too much). At cone 06, 0.5 molar parts of B2O3 are usually needed, whereas at cone 6, only around 0.1-0.2 are required. Reactive glazes often contain much more boron (e.g. 0.4-0.5 at cone 6), these formulations have associated issues with running that contribute to various defects. Almost all frits for low and medium temperatures contain boron as their main melting mechanism.
-Powdered B2O3 cannot be added to a glaze, there is no such insoluble material. It must be done using glaze chemistry. There are many videos and pages here showing how this can be done using your account at Insight-live.com and with Digitalfire Insight.
-Boric oxide itself has no melting point, but a progressive softening and melting range from 300-700C. The crystals begin to break down at 300C, and a series of suboxides are produced with partial melting until full fusion is reached at 700C. Boron frits also behave in this manner.
-Boric oxide is a unique oxide often not fully appreciated for all its qualities. It reacts with whatever is available to behave as both the 'bones' and the 'blood' of glazes (acidic glass former and flux). In some ways, it can thus be considered a low-temperature equivalent of silica (although silica still needs to be present for boron to act as a flux, see below). Because of its dual personality, technicians often are not sure where to place it in the unity formula. If placed with the amphoterics, where chemically it should go, it becomes difficult to compare the formula to others that have no boric oxide.
-Boron has many advantages as a glass-forming oxide. Borosilicate glazes have been the major alternative to lead-based formulations (melting as low as 750C), and thus boron is critical to the ceramic industry. 'Pyrex' ware, for example, is a low expansion, high silica, borosilicate glass.
-The way in which boric oxide combines with oxides like calcia and soda is not as well understood as other systems.
-Its low expansion makes it valuable in preventing crazing. However, each glaze recipe tends to have an optimum amount above which the effect can be reversed and crazing increases (typically 10-14%). This effect is said to be due to the loss of elasticity associated with excess B2O3. Predicting the expansion of high boron glazes can thus be misleading.
-Like silica (in glazes), it does not crystallize on cooling unless significant calcia is present to form calcium borate.
-Boron glazes have less melt fluidity and this has been the major challenge in switching from lead. While many users increased firing temperatures to compensate, this has not fully solved the 'healing' and bubble clearance problems.
-In the sanitaryware industry boron is being used to impart better refire characteristics (where significant reject rates necessitate fix-up and refire). Small amounts (2% from frits) in previously boron-free glazes produce a glaze that does not devitrify, and therefore lose gloss, on second and subsequent firings (see linked article below). In addition, the small boron addition imparts better acid resistance and hardness, lowers melting temperature and reduces thermal expansion.
-In low-temperature glazes, it both substitutes for fluxes of higher-expansion and less potency, and contributes to glass building in lieu of the lower SiO2 percentages present.
-Boron's reactivity helps to form better clay-glaze interfacial zones.
-The action of B2O3 is said to depend upon the ratio of bases to silica existing in the glaze before the addition. If the ratio is greater than 1:2, the glaze will tend toward opalescence and crazing; if less toward clear and transparent.
-Boron can form both borosilicate and borosilite alkali glasses in the same melt, separating (called phase separation). Glasses solidified from such a non-homogeneous melt can have thermal expansion properties that are much lower or higher than expected. Frits very high in boron demonstrate this phenomenon.
-Boron can form a strong eutectic with BaO and it is possible to produce glossy and runny glazes that can solidify below 500C.
-Boron is very important in glass manufacture. It is employed to obtain the low expansion and quick heat transfer necessary for hot-cold cycle endurance. It also imparts corrosion resistance and lower temperature workability (at the expense of working range). Small amounts in ordinary soda-lime glass (1-1.5%) give greater strength, brilliance, durability, thermal shock resistance and protect against the tendency to crystallize during the cool cycle.
-B2O3 can actually be a refractory, frits with very high contents are used in the refractory industry. These frits do not contain SiO2 (depriving boron of a reaction with it to form a borosilicate glass).
How much B2O3 should a glaze have? For low fire glazes, just getting them to melt well is a priority so lots is needed. For stoneware glazes (cone 5 and higher) much less boron is needed. Just enough to melt to the degree needed. This is different for each recipe (e.g. G2934 only needs 0.12 to have a fluid melt, G2926B needs 0.34). Complex eutectics are involved in every recipe. If lithia or zinc are present less boron is needed. If too much boron is present the glaze melts too early and likely will not be suitable for use with decals. That being said, reactive glazes, like those in the Mastering Glazes book, need more boron to get good melt fluidity (and better crystallization on cooling). Likewise, if the glaze is crazing, often the only practical way to get the thermal expansion down is more boron to permit less KNaO and more Al2O3/SiO2.
-Borax and Boracic Acid are both soluble and unsuitable sources for glazes, but fine for frits.
The amazing fluxing power of boron (in borax)

This picture has its own page with more detail, click here to see it.
The two top clay bars contain 15% hydrous borax. At cone 06, a very low temperature, it has already melted and drained out of the bars, running down over the others as a glass.
A frit softens over a wide temperature range

This picture has its own page with more detail, click here to see it.
Raw materials often have a specific melting temperature (or they melt quickly over a narrow temperature range). We can use the GLFL test to demonstrate the development of melt fluidity between a frit and a raw material. On the left we see five flows of boron Ferro Frit 3195, across 200 degrees F. Its melting pattern is slow and continuous: It starts flowing at 1550F (although it began to turn to a glass at 1500F) and is falling off the bottom of the runway by 1750F. The Gerstley Borate (GB), on the other hand, goes from no melting at 1600F to flowing off the bottom by 1625F! But GB has a complex melting pattern, there is more to its story. Notice the flow at 1625F is not transparent, that is because the Ulexite mineral within GB has melted but its Colemanite has not. Later, at 1700F, the Colemanite melts and the glass becomes transparent. Technicians call this melting behaviour "phase transition", that does not happen with the frit.
Frits melt so much better than raw materials

This picture has its own page with more detail, click here to see it.
Feldspar and talc are both flux sources (glaze melters), they are common in all types of stoneware glazes. But their fluxing oxides, Na2O and MgO, are locked in crystal structures that neither melt early or supply other oxides with which they like to interact. The pure feldspar is only beginning to soften at cone 6. Yet the soda frit is already very active at cone 06! As high as cone 6, talc (the best source of MgO) shows no signs of melting activity at all. But a high-MgO frit is melting beautifully at cone 06! The frits progressively soften, starting from low temperatures, both because they have been premelted and have significant boron content. In both, the Na2O and MgO are free to impose themselves as fluxes, actively participating in the softening process.
Boron blue in low fire transparent glazes

This picture has its own page with more detail, click here to see it.
This high-boron high-CaOcone 04 glaze is generating calcium-borate crystals during cool down (called boron-blue). This is a common problem and a reason to control the boron levels in transparent glazes; use just enough to melt it well. If more melt fluidity is needed, decrease the percentage of CaO in favor of a lower melting oxide, that will certainly help. There is a positive: For opaque glazes, this effect can actually enable the use of less opacifier.
Melting range is mainly about boron content

This picture has its own page with more detail, click here to see it.
Fired at 1850. Notice that Frit 3195 is melting earlier. By 1950F, they appear much more similar. Melting earlier can be a disadvantage, it means that gases still escaping as materials in the body and glaze decompose get trapped in the glass matrix. But if the glaze melts later, these have more time to burn away. Glazes that have a lower B2O3 content will melt later, frit 3195 has 23% while Frit 3124 only has 14%).
These common Ferro frits have distinct uses in traditional ceramics

This picture has its own page with more detail, click here to see it.
I used Veegum to form 10 gram GBMF test balls and fired them at cone 08 (1700F). Frits melt really well, they do have an LOI like raw materials. These contain boron (B2O3), it is a low expansion super-melter that raw materials don’t have. Frit 3124 (glossy) and 3195 (silky matte) are balanced-chemistry bases (just add 10-15% kaolin for a cone 04 glaze, or more silica+kaolin to go higher). Consider Frit 3110 a man-made low-Al2O3 super feldspar. Its high-sodium makes it high thermal expansion. It works really well in bodies and is great to make glazes that craze. The high-MgO Frit 3249 (made for the abrasives industry) has a very-low expansion, it is great for fixing crazing glazes. Frit 3134 is similar to 3124 but without Al2O3. Use it where the glaze does not need more Al2O3 (e.g. already has enough clay). It is no accident that these are used by potters in North America, they complement each other well (equivalents are made around the world by others). The Gerstley Borate is a natural source of boron (with issues frits do not have).
These two frits have one difference in the chemistry: Al2O3.

This picture has its own page with more detail, click here to see it.
These two boron frits (Ferro 3124 left, 3134 right) have almost the same chemistry. But there is one difference: The one on the right has no Al2O3, the one on the left has 10%. Alumina plays an important role (as an oxide that builds the glass) in stiffening the melt, giving it body and lowering its thermal expansion, you can see that in the way these flow when melting at 1800F. The frit on the right is invaluable where the glaze needs clay to suspend it (because the clay can supply the Al2O3). The frit on the left is better when the glaze already has plenty of clay, so it supplies the Al2O3. Of course, you need to be able to do the chemistry to figure out how to substitute these for each other because it involves changing the silica and kaolin amounts in the recipe also.
Ceramic Oxide Periodic Table

Pretty well all common traditional ceramic base glazes are made from less than a dozen elements (plus oxygen). Go to the full picture of this table and click or tap each of the oxides to learn more (on its page at digitalfire.com). When materials melt, they decompose, sourcing these elements in oxide form. The kiln builds the glaze from them, it does not care what material sources what oxide (assuming, of course, that all materials do melt or dissolve completely into the melt to release those oxides). Each of these oxides contributes specific properties to the glass. So, you can look at a formula and make a good prediction of the properties of the fired glaze. And know what specific oxide to increase or decrease to move a property in a given direction (e.g. melting behavior, hardness, durability, thermal expansion, color, gloss, crystallization). And know about how they interact (affecting each other). This is powerful. A lot of ceramic materials are available, hundreds - that is complicated when individual materials source multiple oxides. Viewing a glaze as a simple unity formula of ceramic oxides is just simpler.
Before spending time trying online recipes, take a minute to do a sanity check on them

This picture has its own page with more detail, click here to see it.
This is a cone 6 GLFL test to compare melt-flow between a matte recipe, found online at a respected website, and a glaze we use often. Yes, it is matte. But why? Because it is not melted! Matte glazes used on functional surfaces need to melt well, they should flow like a glossy glaze. Even though this recipe has 40% nepheline syenite, lots of dolomite and calcium carbonate it is not melting. Yes, these are powerful fluxes, but at cone 10, not cone 6! To melt a cone 6 glaze boron, zinc or lithia are needed. Boron is the most common and best general-purpose melter for potters (it comes mainly in frits, Gerstley borate). The concept of a limit recipe applies here, the idea of eye-balling a recipe and quickly assessing if it is ridiculous or not.
Slow cooling vs. fast cooling on a cone 6 transparent glaze

This picture has its own page with more detail, click here to see it.
These are the inside uppers on two mugs made from the same clay with the same clear glaze. The one on the left was fired in a large electric kiln full of ware (thus it cooled relatively slowly). The one on the right was in a test kiln and was cooled rapidly. This glaze contains 40% Ferro Frit 3134 so there is plenty of boron and plenty of calica to grow the borosilicate crystals that cause the cloudiness in the glass. But in the faster cooling kiln they do not have time to grow.
Al2O3 in glazes make them durable and wear resistant

This picture has its own page with more detail, click here to see it.
The cone 6 glazes on the left have double the boron of those on the right so they should be melting much more. But they flow less because they have much higher Al2O3 and SiO2 contents. This effect renders them milky white vs. the transparent of those on the right. Why? Because G and H are trapping micro-bubbles because of the increased viscosity of the melt. In spite of this, the two on the left do fire almost transparent when applied to ware, they have enough fluidity to shed most of the bubbles when in a thin layer. The ones on the right are too fluid, they will run excessively on ware unless applied thinly. The sweet-spot is a little more fluidity than those on the left. But there is another very important factor: Durability. The increased Al2O3 in G and H make them fire harder, more resistant to abrasion. The added SiO2 adds resistance to leaching.
Why does Gerstley Borate melt in two stages? Because it is two minerals.
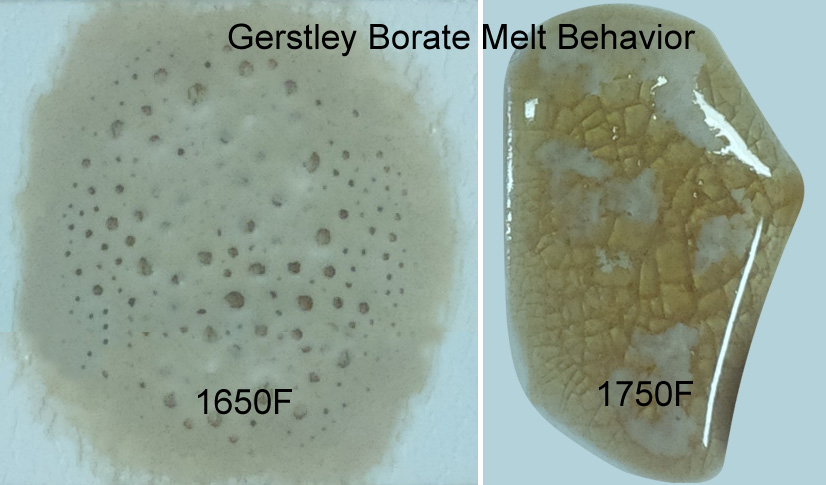
This picture has its own page with more detail, click here to see it.
The ulexite in Gerstley Borate melts first, producing an opaque fired glass having the unmelted (and still gassing) particles of colemanite suspended in it. By 1750F the colemanite is almost melted also. Boron-containing frits, by contrast, soften slowly over a wide temperature range and gradually spread and melt. Not surprisingly they produce a more stable glaze (albeit often less interesting visually without additives e.g. titanium, rutile). These behavior contributes to phase changes in fired glazes that contribute to variegation.
Your boron glaze might melt alot earlier than you think

This picture has its own page with more detail, click here to see it.
The porcelain mug on the left is fired to cone 6 with G2926B clear glossy glaze. This recipe only contains 25% boron frit (0.33 molar of B2O3). Yet the mug on the right (the same clay and glaze) is only fired to cone 02 yet the same glaze is already well melted! What does this mean? Industry avoids high boron glazes (they consider 0.33 to be high boron) because this early melting behavior means gases cannot clear before the glaze starts to melt (causing surface defects). For this reason, fast fire glazes melt much later. Yet many middle temperature reactive glazes in use by potters have double the amount of B2O3 that this glaze has!
Does adding boron alone always increase glaze melt?

This picture has its own page with more detail, click here to see it.
Boron (B2O3) is like silica, but it is also a flux. Frits and Gerstley Borate supply it to glazes. In this test, I increased the amount of boron from 0.33 to 0.40 (using the chemistry tools in my insight-live.com account). I was sure that this would make the glaze melt more and have less of a tendency to craze. But as these GBMF tests for melt flow (10 gram GBMF test balls melted on porcelain tiles) show, that did not happen. Why? I am guessing that to get the effect B2O3 has to be substituted, molecule for molecule for SiO2 (not just added to the glaze).
An ultra-clear brilliantly-glossy cone 6 clear base glaze? Yes!

This picture has its own page with more detail, click here to see it.
I am comparing 6 well known cone 6 fluid melt base glazes and have found some surprising things. The top row are 10 gram GBMF test balls of each melted down onto a tile to demonstrate melt fluidity and bubble populations. Second, third, fourth rows show them on porcelain, buff, brown stonewares. The first column is a typical cone 6 boron-fluxed clear. The others add strontium, lithium and zinc or super-size the boron. They have more glassy smooth surfaces, less bubbles and would should give brilliant colors and reactive visual effects. The cost? They settle, crack, dust, gel, run during firing, craze or risk leaching. Out of this work came the G3806E and G3806F.
In pursuit of a reactive cone 6 base that I can live with

This picture has its own page with more detail, click here to see it.
These GLFL tests and GBMF tests for melt-flow compare 6 unconventionally fluxed glazes with a traditional cone 6 moderately boron fluxed (+soda/calcia/magnesia) base (far left Plainsman G2926B). The objective is to achieve higher melt fluidity for a more brilliant surface and for more reactive response with colorant and variegator additions (with awareness of downsides of this). Classified by most active fluxes they are:
G3814 - Moderate zinc, no boron
G2938 - High-soda+lithia+strontium
G3808 - High boron+soda (Gerstley Borate based)
G3808A - 3808 chemistry sourced from frits
G3813 - Boron+zinc+lithia
G3806B - Soda+zinc+strontium+boron (mixed oxide effect)
This series of tests was done to choose a recipe, that while more fluid, will have a minimum of the problems associated with such (e.g. crazing, blistering, low run volatility, susceptibility to leaching). As a final step the recipe will be adjusted as needed. We eventually evolved the G3806B, after many iterations settled on G3806E or G3806F as best for now.
The same glaze fires very differently depending on kiln cooling rate

This picture has its own page with more detail, click here to see it.
Both mugs use the same cone 6 oxidation high-iron (9%), high-boron, fluid melt glaze. Iron silicate crystals have completely invaded the surface of the one on the left, turning the gloss surface into a yellowy matte. Why? Multiple factors. This glaze does not contain enough iron to guarantee crystallization on cooling. When cooled quickly it fires the ultragloss near-black on the right. As cooling is slowed at some point the iron will begin to precipitate as small scattered golden crystals (sometimes called Teadust or Sparkles). As cooling slows further the number and size of these increases. Their maximum saturation is achieved on the discovery, usually by accident, of the likely narrow temperature range they form at (normally hundreds of degrees below the firing cone). Potters seek this type of glaze but industry avoids it because of difficulties with consistency.
High B2O3 imparts better melt fluidity, but also fewer micro-bubbles

This picture has its own page with more detail, click here to see it.
A cone 6 firing. The glaze on the left has a B2O3 molar content of 0.54 whereas the one on the right has 0.64 (other oxide levels are the same). This is triple the typical amount of boron in a cone 6 glaze, the result is obvious: High melt fluidity for both. But G3904A has a significant difference: The flow is more transparent because of the lower micro-bubble population. It's melt better enables the bubbles to pass, exit and the surface to heal. Why don't all glazes use more boron? Cost. Frits are expensive and they are the best source of boron. There is also a cost to durability (although mitigated when there is plenty of Al2O3 and SiO2 present, as is the case here). These recipes were part of a project to fix a recipe where the potter mistakenly used Frit 3134 instead of 3124 when mixing a large batch of glaze. I calculated how much kaolin and silica to add to bring the chemistry back into line with the original. This was possible because frit 3134 chemistry is an approximate oxide-subset of 3124. The resultant glaze is potentially better than the original.
Links
| Articles |
G1916M Cone 06-04 transparent glaze
This is a frit based boron glaze that is easily adjustable in thermal expansion, a good base for color and a starting point to go on to more specialized glazes. |
| URLs |
http://www.ceramicindustry.com/CDA/ArticleInformation/features/BNP__Features__Item/0,2710,123649,00.html
Using borates in brick and roofing tile |
| URLs |
http://www.ceramicindustry.com/articles/borates-a-new-flux-for-glossy-glazes
Formulating borate glazes to premit refire without loss in gloss |
| URLs |
http://m.youtube.com/user/tonywilliamhansen
YouTube channel for Tony Hansen |
| Glossary |
Medium Temperature
These are stoneware glazes that fire in the range of 1200C (2200F). They often contain boron to assist with melting. |
| Glossary |
Boron Frit
Most ceramic glazes contain B2O3 as the main melter. This oxide is supplied by great variety of frits, thousands of which are available around the world. |
| Glossary |
Borate
Borate glazes, those fluxed with the oxide B2O3, are the most common type used in ceramic industry and hobby for low and medium temperatures. |
| Glossary |
Ceramic Oxide
In glaze chemistry, the oxide is the basic unit of formulas and analyses. Knowledge of what materials supply an oxide and of how it affects the fired glass or glaze is a key to control. |
| Glossary |
Limit Formula
A way of establishing guideline for each oxide in the chemistry for different ceramic glaze types. Understanding the roles of each oxide and the limits of this approach are a key to effectively using these guidelines. |
| Glossary |
Flux
Fluxes are the reason we can fire clay bodies and glazes in common kilns, they make glazes melt and bodies vitrify at lower temperatures. |
| Materials |
Gerstley Borate
Gerstley Borate was a natural source of boron for ceramic glazes. It was plastic and melted clear at 1750F. Now we need to replace it. How? |
| Materials |
Colemanite
A natural source of boron that melts at a very low temperature. |
| Materials |
Boric Acid
|
| Materials |
Borax Decahydrate
|
| Materials |
Boric Oxide
|
| Materials |
Borax Pentahydrate
|
| Materials |
Ulexite
A natural source of boron, it melts at a very low temperature to a clear glass. |
| Materials |
Boron Frits
|
| Materials |
Matrix Frit B40-C
|
| Oxides | PbO - Lead Oxide |
Mechanisms
| Glaze Color | Low fire transparent glazes employing boron frits, which have CaO and lack alumina, will have opalescent blue cloudy effects from the formation of calcium borate crystals. These 'boron blue' glazes work well visually on terra cotta bodies. These crystals do not form well if there is adequate alumina to stiffen the melt. |
|---|
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
