| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
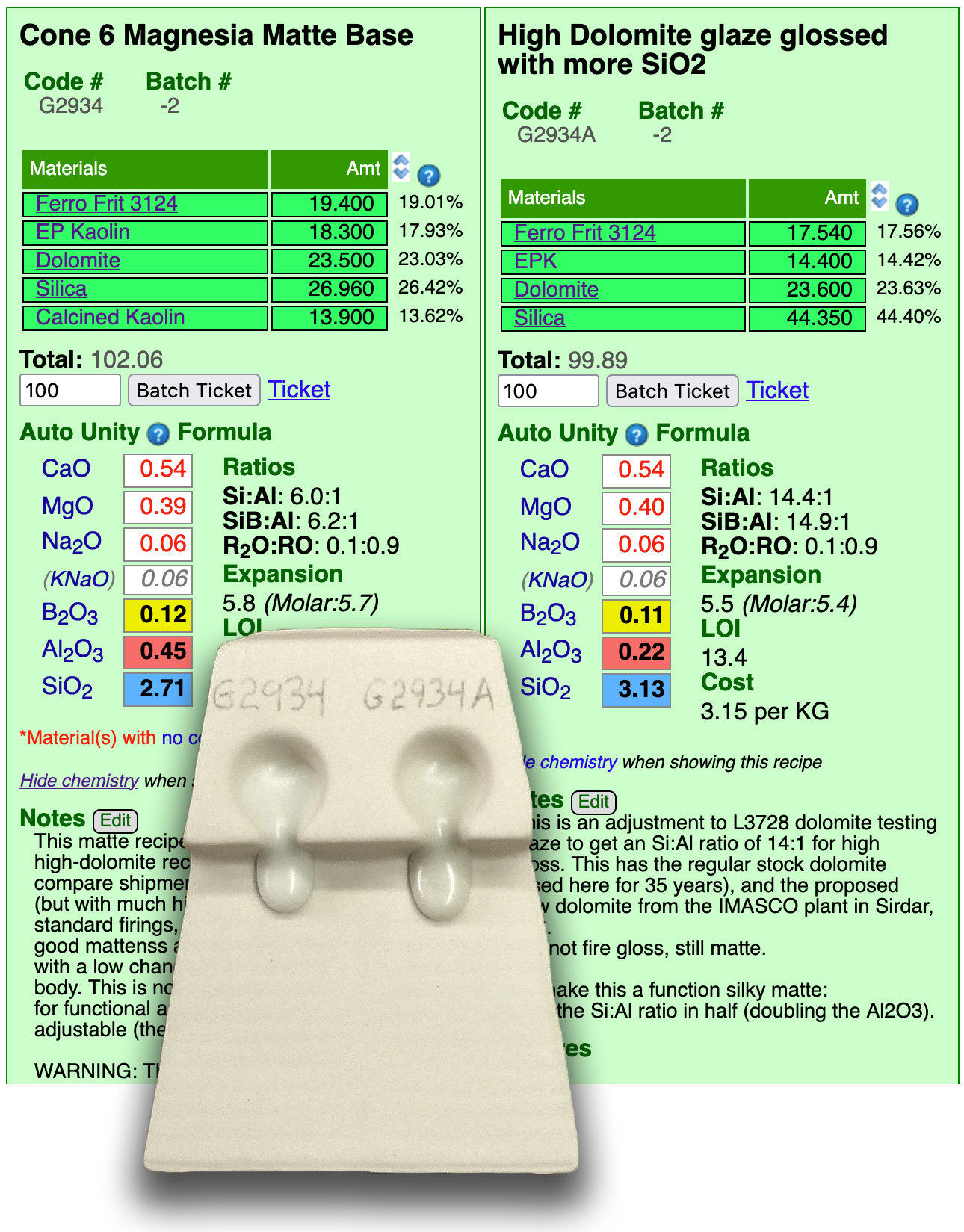
G2934 plus 10% silica actually flows more
Until now, I thought that magnesia matte glazes need high Al2O3 and a low Si:Al ratio. But this melt flow test suggests otherwise. The cone 6 glaze on the left (A) is close to my usual target of 0.4 MgO, 0.1 B2O3 and a ratio of 6:1. It melts well but does not run. The typical MgO mechanism of phase separation and micro-wrinkling of the surface thrives here. But glaze B has a super-high ratio of 14:1, very low Al2O3 and a super-high percentage of silica in the recipe. Yet it runs better and is still matte (although slightly less so). How?
It seems I stumbled onto a shift between two different types of matte surfaces. Dropping the Al2O3 to 0.22 and spiking the SiO2 has moved from an alumina matte to a silica matte. B-mattness is likely caused by saturation and devitrification. Even though SiO2 is a glass former, it needs enough "alumina-scaffolding" to stay in a stable, disordered glass state. By cutting the Al2O3 in half and creating a massive surplus of SiO2, I’ve weakened the "glue" that keeps the MgO and CaO busy in the glass matrix (so they find each other and precipitate out as crystals), likely as diopside (CaMgSi2O6) or enstatite (MgSiO3). High-magnesia/low-alumina melts can also undergo phase separation into silica-rich and flux-rich liquids (like oil and vinegar), also creating a "micro-wrinkled" or mottled texture.
The second one flows more because alumina is incredibly refractory, creating viscous melts. By dropping it so much, we have removed the "brakes." Even with added silica, the balance has shifted in favor of the fluxes.
Related Pictures
Calcia vs Magnesia matte - Different mechanisms

This picture has its own page with more detail, click here to see it.
This melt flow test was done at cone 6+ (without slow cooling) to demonstrate the difference in melt viscosity between a calcia matte (left) and a magnesia matte (right). In simplest terms, the former depends on a fluid melt to provide the needed mobility for tiny crystals to form during cooling, those crystals scatter the light and soften the surface to produce the matte effect. The latter requires a stiffer melt to help prevent leveling during cooling and host phase separation to produce a surface that scatters light.
Videos
Links
| Glossary |
Calcia Matte
Calcia matte ceramic glazes are “crystal mattes” while magnesia mattes are “microstructure mattes”. They have a smoother surface but can also have finely textured, even frosty, feathery or sugary surfaces, depending cooling and crystalliztion. |
| Glossary |
Magnesia Matte
Magnesia matte ceramic glazes are “microstructure mattes” while calcia mattes are “crystal mattes”. They have a micro-wrinkle surface that forms from a high viscosity melt and microscopic phase separation, both of which prevent levelling on freezi |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy