| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
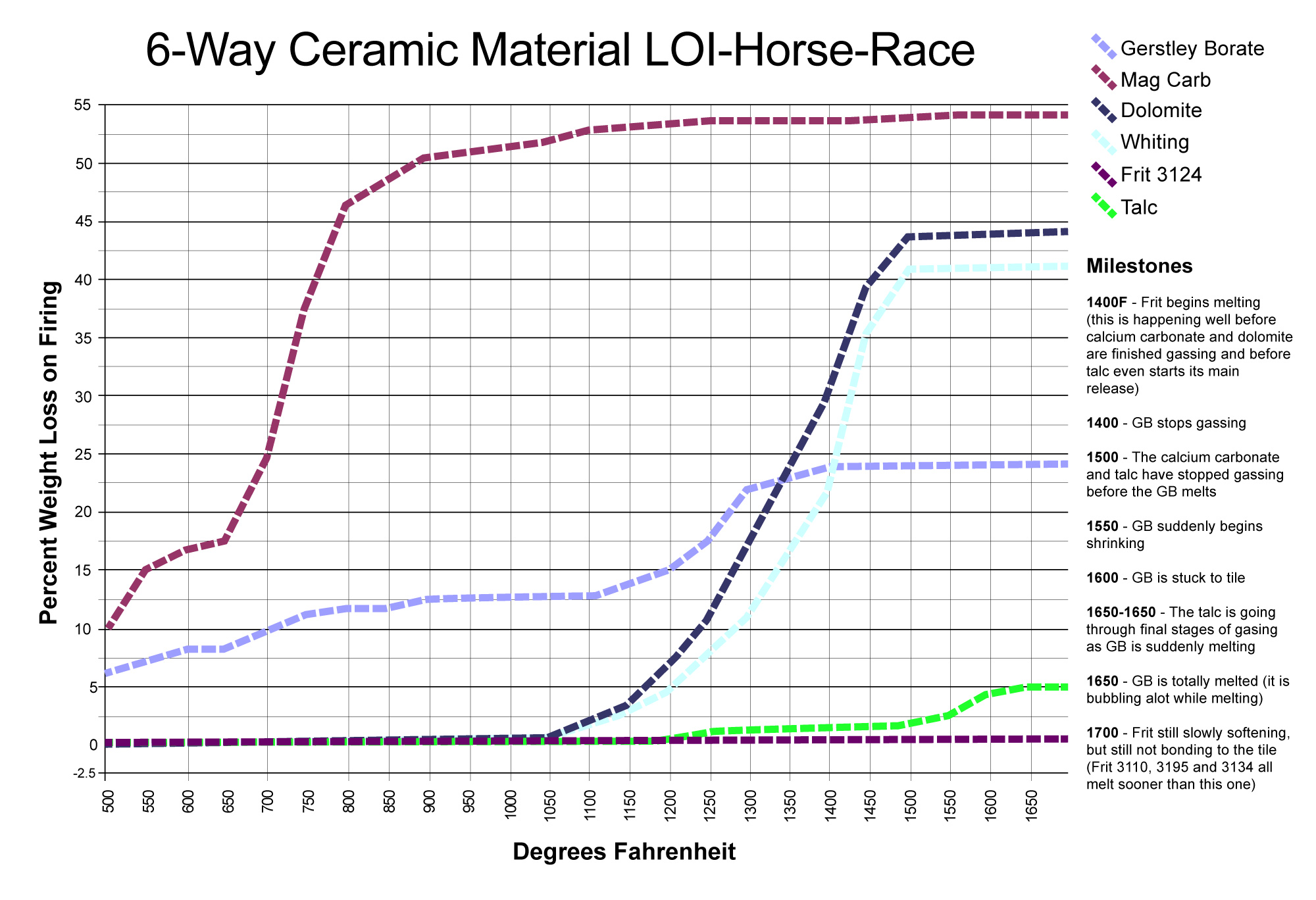
LOI horse race with surprising winners
This chart compares the decompositional off-gassing (% weight Loss on Ignition) behavior of six glaze materials as they are heated through the range 500-1700F. It is amazing how much weight some can lose on firing - for example, 100 grams of calcium carbonate generates 45 grams of CO2! This chart is a reminder that some late gassers overlap early melters. That is a problem. The LOI of these materials can affect glazes (causing bubbles, blisters, pinholes, crawling). Talc is an example: It is not finished gassing until 1650F, yet many fritted glazes have already begun melting by then. Even Gerstley Borate, a raw material, begins to melt while talc is barely finished gassing. Dolomite and calcium carbonate Other materials also create gases as they decompose during glaze melting (e.g. clays, carbonates, dioxides).
Related Pictures
Talc was making this glaze "puffy" - here is how I fixed it.

This picture has its own page with more detail, click here to see it.
The recipe on the right employs talc to source the MgO needed to create a silky matte texture. However, talc is a late gasser, potentially able to produce micro-bubbles in the glaze after it has begun melting. The reduction in the definition of contour edges on the tile in front and the reduction in melt fluidity tipped me off. Using my account at insight-live.com I did the calculations needed to source the MgO using dolomite instead, producing recipe G3933E. While dolomite has a far higher LOI than talc it starts releasing the gasses of its decomposition much earlier and finishes well before talc. The mug in the back confirmed my suspicions, firing with a much smoother surface (and with far better definition of the incising).
Videos
Links
| Materials |
Light Magnesium Carbonate
A refractory feather-light white powder used as a source of MgO and matting agent in ceramic glazes |
| Materials |
Ferro Frit 3124
A commonly available calcium borosilicate frit. |
| Materials |
Calcium Carbonate
In ceramics, calcium carbonate is primarily a source of CaO in raw stoneware and porcelain glazes. |
| Materials |
Talc
A source of MgO for ceramic glazes, a flux or thermal expansion additive in clay bodies, also used in the manufacture of cordierite. |
| Materials |
Dolomite
An inexpensive source of MgO and CaO for ceramic glazes, also a highly refractory material when fired in the absence of reactant fluxes. |
| Materials |
Frit
Frits are made by melting mixes of raw materials, quenching the melt in water, grinding the pebbles into a powder. Frits have chemistries raw materials cannot. |
| Materials |
Gerstley Borate
Gerstley Borate was a natural source of boron for ceramic glazes. It was plastic and melted clear at 1750F. Now we need to replace it. How? |
| Temperatures | Gerstley Borate stops gassing (760-) |
| Temperatures | Calcium carbonate, talc finished gassing (815-) |
| Temperatures | Talc has finished gassing (900-) |
| Temperatures | Decarbonation (200-1000) |
| Temperatures | Comparison of frit melts at 1650F (900C) (1650-) |
| Temperatures | Common frits begin melting (760-) |
| Articles |
Firing: What Happens to Ceramic Ware in a Firing Kiln
Understanding more about changes taking place in the ware at each stage of a firing helps tune the curve and atmosphere to produce better ware |
| Glossary |
Pinholing
Pinholing is a common surface defect that occurs with ceramic glazes. The problem emerges from the kiln and can occur erratically in production. |
| Glossary |
LOI
Loss on Ignition is a number that appears on the data sheets of ceramic materials. It refers to the amount of weight the material loses as it decomposes to release water vapor and various gases during firing. |
| Glossary |
Glaze Bubbles
Suspended micro-bubbles in ceramic glazes affect their transparency and depth. Sometimes they add to to aesthetics. Often not. What causes them and what to do to remove them. |
| Oxides | LOI - Loss on Ignition |
| Troubles |
Glaze Blisters
Questions and suggestions to help you reason out the real cause of ceramic glaze blistering and bubbling problems and work out a solution |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy