| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Calcium Carbonate
Alternate Names: Carbonate of Lime, Whiting, Aragonite, Calcite, CaCO3
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 56.10% | 1.00 | |
| CO2 | 43.90% | n/a | |
| Oxide Weight | 56.10 | ||
| Formula Weight | 100.00 | ||
Notes
Whiting has traditionally been a source of CaO in raw glazes and glass (however whitings also typically contain some dolomite as a contaminant). Whiting is generally inexpensive and there is a large calcium carbonate industry worldwide for non-ceramic uses of this mineral. Well-known deposits are the chalk cliffs of England, France and Belgium. Marble and calcite ores are abundant in many places.
Inexpensive nonceramic grades of whiting tend to lack the quality and consistency needed for use in glazes (especially for industrial use). Also, whiting produces a very large volume of gases while decomposing, it loses more than 40% by weight. While these gases should be gone well before 1100C (and therefore should not disturb the glaze melt), in low or fast fire they can contribute to imperfections and faults in the glaze surface. With the advent of faster firing schedules in recent years, whiting has been replaced by wollastonite and frits as a source of CaO in many applications (CaO oxide is advantageous in fast fire because it does not lower the melting point as much as the alkalies). Since LOI is a good indicator of variation in chemistry it may be practical to do an LOI test on shipments by firing a specimen of powder in a thin bisqued bowl to confirm the consistency of shipments.
There are many alternate no-LOI sources of CaO (e.g. wollastonite, frits) and incorporating one of them to source the CaO instead is a classic application of glaze chemistry calculations. However, remember that CaO is not an active melter below about cone 8, so particle size can make a big difference in its willingness to enter the glaze melt. A 325 mesh material could create a glossy glaze whereas a 200 mesh could create a silky matte, exclusively because of the difference in particle size. A 325 mesh material may have a mean particle size of only 10 microns (or even less), whereas a 200 mesh grade might be two or three times that (yet both powders feel the same).
In glazes that contain both calcium carbonate and silica, it is preferable to source as much as possible of the SiO2 from wollastonite instead. This is because the SiO2 in wollastonite is taken into solution in the melt much more easily than from highly refractory quartz particles.
In low-fire bodies, calcium carbonate is sometimes added in small amounts as a filler to reduce fired shrinkage and act as a whitener. It is also common to see 5% whiting included in porous earthenware body recipes to prevent moisture expansion (which causes glazes to craze).
Related Information
Example thermogravimetric analysis of calcium carbonate

This picture has its own page with more detail, click here to see it.
What happens when a limestone clay mix is fired to cone 6?

This picture has its own page with more detail, click here to see it.
The top bar is a mix of calcium carbonate and a middle temperature stoneware clay (equal parts). On removal from the kiln it appears and behaves like a normal stoneware clay body, hard and strong. However, pour water on it and something incredible happens: in a couple of minutes it disintegrates (as it rehydrates). And generates lots of heat as it does so.
Calcium carbonate and dolomite are refractory when used pure

This picture has its own page with more detail, click here to see it.
Examples of calcium carbonate (top) and dolomite (both mixed with 25% bentonite to make them plastic enough to make a test bars). They are fired to cone 9. Both bars are porous and refractory, even powdery. However, put either of these in a mix with other ceramic minerals and they interact strongly to become fluxes.
CaO is a strong flux but it can cause crazing

This picture has its own page with more detail, click here to see it.
2, 5, 10, 15% calcium carbonate added to Ravenscrag Slip on a buff stoneware fired at cone 10R. It gets progressively glossier toward 15%, crazing starts at 10% (test by Kat Valenzuela). Adding a flux only reduces the SiO2 and Al2O3, this pushes the thermal expansion upwards. 5% is actually sufficient. An alternative would be to use wollastonite, it supplies SiO2 also.
Comparing two brands of calcium carbonate

This picture has its own page with more detail, click here to see it.
This GLFL test compares the melt flow, at cone 6, of two glaze recipes containing the calcium carbonates (of which they make up 27%). Notice the amount of bubbles (due to the high LOI, or loss on ignition). The 3HX is flowing a little more. This could be because of a difference their proportions of dolomite and calcium carbonate minerals, the individual mineral purities or the particle size (or all three). Whatever the case, 3HX will make a glaze flow a slightly better.
Calcium carbonate and glaze bubbles

This picture has its own page with more detail, click here to see it.
The two cone 04 glazes on the right have the same chemistry but the center one sources it's CaO from 12% calcium carbonate and ulexite (the other from Gerstley Borate). The glaze on the far left? It is almost bubble free yet it has 27% calcium carbonate. Why? It is fired to cone 6. At lower temperatures carbonates and hydrates (in body and glaze) are more likely to form gas bubbles because that is where they are decomposing (into the oxides that stay around and build the glass and the ones that are escaping as a gas). By cone 6 the bubbles have had lots of time to clear.
The difference between these low fire transparents: Gerstley Borate vs. Ulexite

This picture has its own page with more detail, click here to see it.
Left: Worthington Clear cone 04 glaze (A) uses Gerstley Borate to supply the B2O3 and CaO. Right: A substitute using Ulexite and 12% calcium carbonate (B). The degree of melting is the same but the gassing of the calcium carbonate has disrupted the flow of B. Gerstley Borate gasses also, but does so at a stage in the firing that does not disrupt this recipe. However, as a glaze, B does not gel and produces a clearer glass. A further adjustment to source CaO from non-gassing wollastonite would likely improve it.
Test bar made from 20% bentonite and 80% calcium carbonate

This picture has its own page with more detail, click here to see it.
It fumes a glassy glaze onto nearby test bars at cone 10R. This fumed glaze layer on the other bars is thick enough to craze and is transparent and glossy. Any ideas why this happens? Please let me know.
GR10-B Ravenscrag transparent glaze (with 10 talc)

This picture has its own page with more detail, click here to see it.
Because this glaze employs 10% dolomite instead of 10% calcium carbonate it has a lower thermal expansion and is less likely to craze. While the dolomite is contributing MgO, which normally mattes glazes, there is not enough to do it here.
The difference between dolomite and calcium carbonate in a glaze

This picture has its own page with more detail, click here to see it.
These glaze cones are fired at cone 6 (without slow cooling) and have the same recipe: 20 Frit 3134, 21 EP Kaolin, 27 calcium carbonate, 32 silica. The difference: The one on the left uses dolomite instead of calcium carbonate. Notice how the MgO from the dolomite mattes the surface (without slow cooling) whereas the CaO from the calcium carbonate produces a brilliant gloss (because it needs slow cooling).
The perfect storm of high surface tension and high LOI: Blisters.

This picture has its own page with more detail, click here to see it.
An example of how calcium carbonate can cause blistering as it decomposes during firing. This is a cone 6 Ferro Frit 3249 based transparent (G2867) with 15% calcium carbonate added (there is no blistering without it). Calcium carbonate has a very high loss on ignition (LOI) and for this glaze, the gases of its decomposition are coming out at the wrong time. While there likely exists a firing schedule that takes this into account and could mature it to a perfect surface, the glaze is high in MgO, it has a high surface tension. That is likely enabling bubbles to form and hold better.
LOI horse race with surprising winners
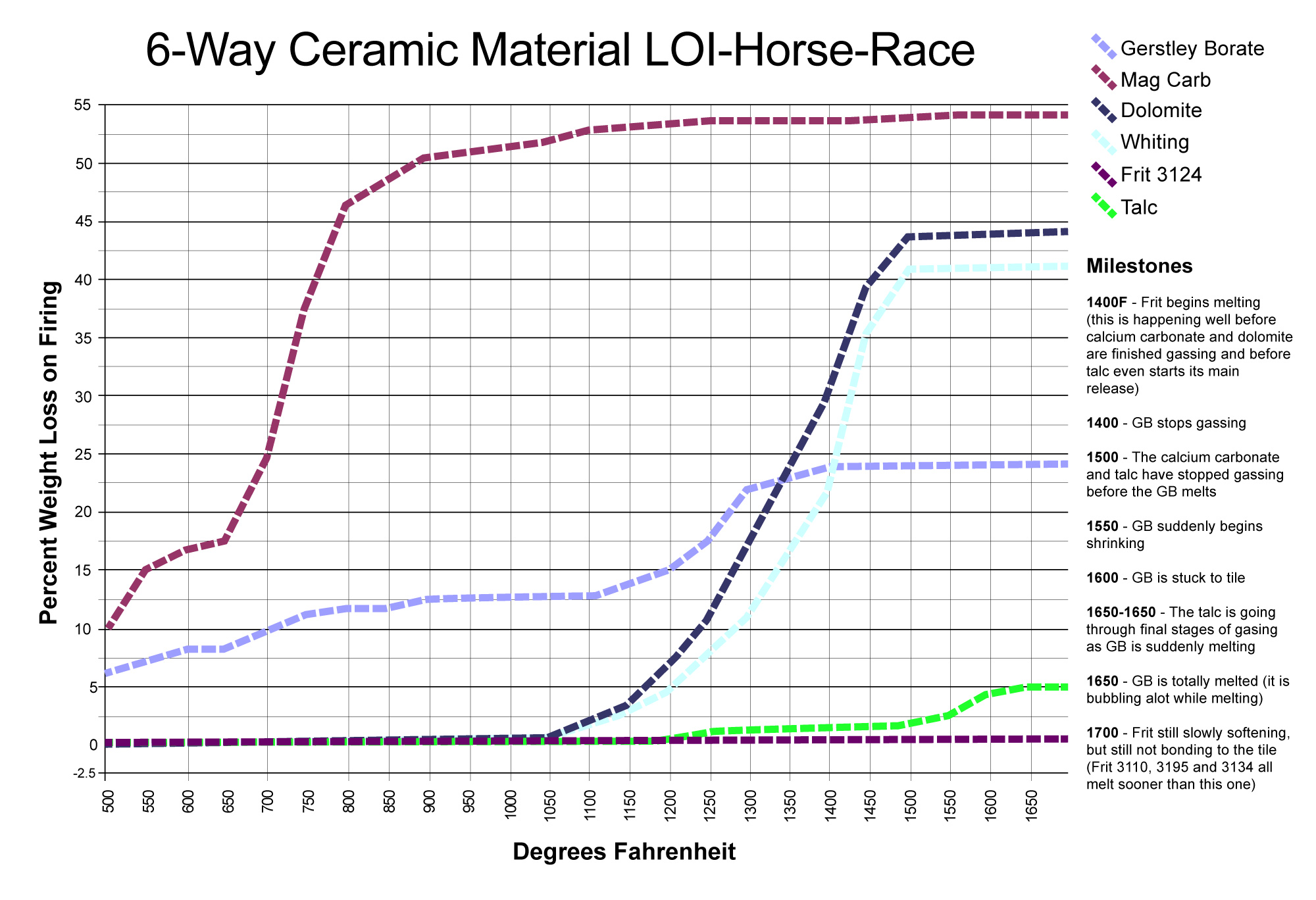
This picture has its own page with more detail, click here to see it.
This chart compares the decompositional off-gassing (% weight Loss on Ignition) behavior of six glaze materials as they are heated through the range 500-1700F. It is amazing how much weight some can lose on firing - for example, 100 grams of calcium carbonate generates 45 grams of CO2! This chart is a reminder that some late gassers overlap early melters. That is a problem. The LOI of these materials can affect glazes (causing bubbles, blisters, pinholes, crawling). Talc is an example: It is not finished gassing until 1650F, yet many fritted glazes have already begun melting by then. Even Gerstley Borate, a raw material, begins to melt while talc is barely finished gassing. Dolomite and calcium carbonate Other materials also create gases as they decompose during glaze melting (e.g. clays, carbonates, dioxides).
Orange-peel or pebbly glaze surface. Why?

This picture has its own page with more detail, click here to see it.
This is a cone 10 glossy glaze. It has the chemistry that suggests it should be crystal clear and smooth. But there are multiple issues with the materials supplying that chemistry: Strontium carbonate, talc and calcium carbonate. Each has a significant LOI and produces gases decomposition. When the gases need to come out at the wrong time it turns the glaze into a Swiss cheeze of micro-bubbles. A study to isolate which of these three materials is the problem might make it possible to adjust the firing to accommodate it. But probably not. The most obvious solution is to just use non-gassing sources MgO, SrO, CaO and BaO (which will require some calculation). There is a good reason to do this: The glaze contains some boron frit, that is likely kick-starting melting much earlier than a standard raw-material-only cone 10 glaze. That fluid melt may not only be trapping gases from the body but creating a perfect environment to trap all the bubbles coming out of those carbonates and talc. All of this being said, a drop and hold firing schedule could also smooth it out a lot.
Links
| Materials |
Lime
|
| Materials |
Wollastonite
|
| Materials |
Vicron Whiting
|
| Materials |
Marblewhite Whiting
|
| Materials |
Limestone
|
| Temperatures | Calcium carbonate decomposition (750-1000) |
| Hazards |
Calcium Carbonate Toxicology
|
| Typecodes |
Generic Material
Generic materials are those with no brand name. Normally they are theoretical, the chemistry portrays what a specimen would be if it had no contamination. Generic materials are helpful in educational situations where students need to study material theory (later they graduate to dealing with real world materials). They are also helpful where the chemistry of an actual material is not known. Often the accuracy of calculations is sufficient using generic materials. |
| Typecodes |
Flux Source
Materials that source Na2O, K2O, Li2O, CaO, MgO and other fluxes but are not feldspars or frits. Remember that materials can be flux sources but also perform many other roles. For example, talc is a flux in high temperature glazes, but a matting agent in low temperatures ones. It can also be a flux, a filler and an expansion increaser in bodies. |
| Oxides | CaO - Calcium Oxide, Calcia |
| Minerals |
Limestone
Limestone forms by sedimentation, of coral and shells (biological limestone) or by the precipitation |
| Minerals |
Aragonite
A calcium carbonate mineral with a different crystal structure than calcite. |
| Minerals |
Calcite
The pure calcium carbonate crystal mineral. |
Data
| Hardness (Moh) | 3.01 |
|---|---|
| Frit Softening Point | 825C D |
| Density (Specific Gravity) | 2.80 |
Mechanisms
| Glaze Opacifier | High calcium coupled with lower alumina will favour the formation of calcium silicate crystals during cooling. This mechanism promotes opacity. |
|---|
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
