| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
LOI (Loss on Ignition)
Notes
-The LOI summarizes the oxides within a raw material that burn away as "products of decomposition" during firing. Some material producers separate the different components of weight lost during firing as H2O, CO2, SO3, even pure carbon, etc. Since products of decomposition escape as gases during firing, fired glazes do not have an LOI, it is thus not expressed as part of the oxide chemistry.
-The formula weight of a material having an LOI is derived as the sum of the products of oxide weights and their amounts divided by (100-LOI)/100. For example, if the weight of the ceramic oxides (those that survive firing) is 90 and the LOI is 5%, then the total formula weight is 90/((100-5)/100) = 94.74.
Desktop INSIGHT MDT dialog showing kaolin LOI

This picture has its own page with more detail, click here to see it.
The LOI appears below the material name and alternative names (beside the weight). The formula that goes with that LOI is the bold numbers in the blanks beside the oxide names on the right.
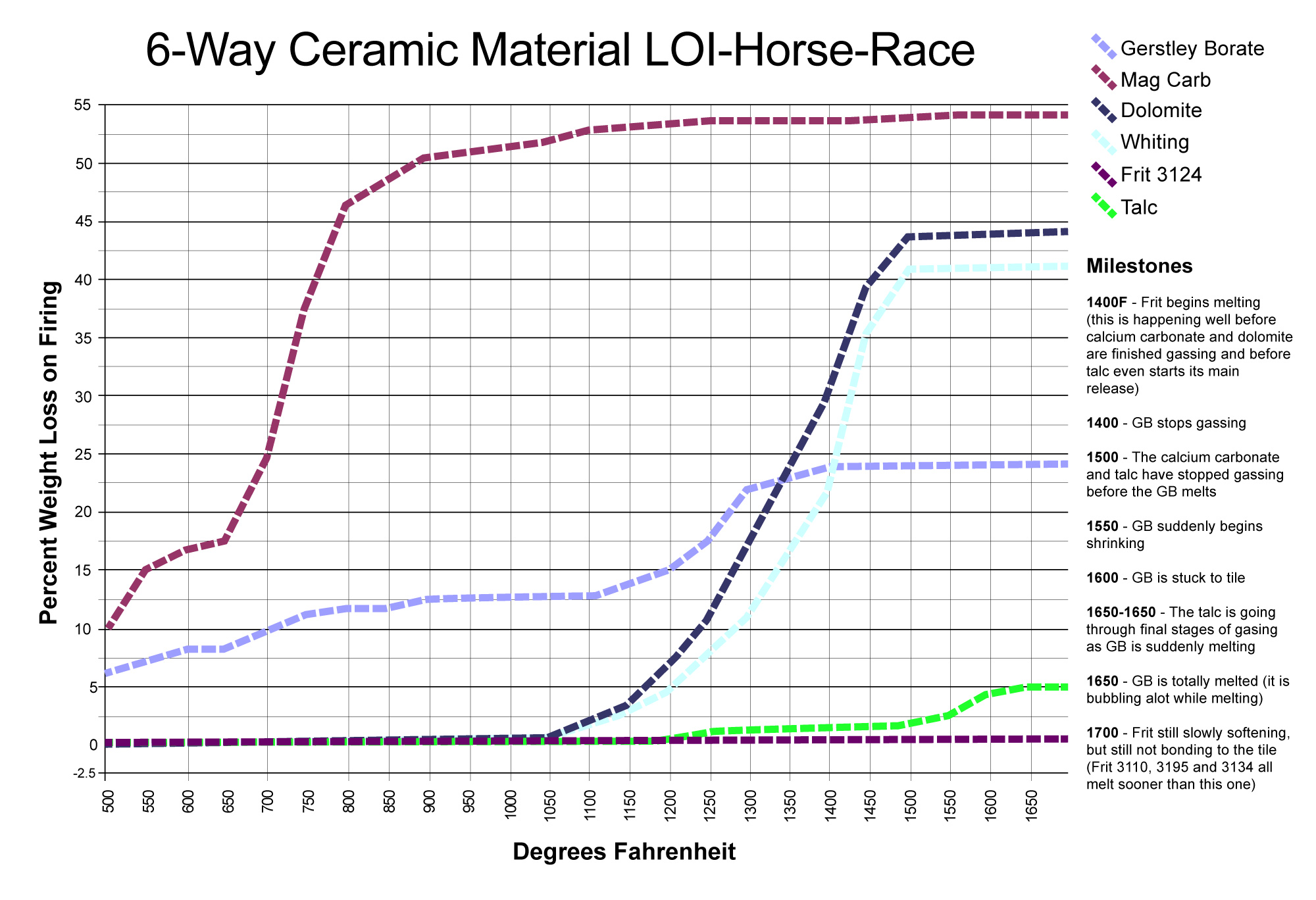
LOI horse race with surprising winners
This picture has its own page with more detail, click here to see it.
This chart compares the decompositional off-gassing (% weight Loss on Ignition) behavior of six glaze materials as they are heated through the range 500-1700F. It is amazing how much weight some can lose on firing - for example, 100 grams of calcium carbonate generates 45 grams of CO2! This chart is a reminder that some late gassers overlap early melters. That is a problem. The LOI of these materials can affect glazes (causing bubbles, blisters, pinholes, crawling). Talc is an example: It is not finished gassing until 1650F, yet many fritted glazes have already begun melting by then. Even Gerstley Borate, a raw material, begins to melt while talc is barely finished gassing. Dolomite and calcium carbonate Other materials also create gases as they decompose during glaze melting (e.g. clays, carbonates, dioxides).
LOI test of common materials flags copper carbonate

This picture has its own page with more detail, click here to see it.
These are pure samples (with 2% binder added) of (top left to bottom right) strontium carbonate, nepheline syenite, cobalt carbonate, manganese dioxide, bentonite (in bowl), 6 Tile kaolin, New Zealand kaolin and copper carbonate. I am firing them at 50F increments from 1500F and weighing to calculate loss on ignition for each. I want to find out at what temperature they are gassing (and potentially bubble-disrupting the glaze they are in or under). Notice how the copper is fuming and spitting black specks on the shelf, this happens right around 1500F (these stains on the shelf darkened considerably when the kiln was fired higher). Yet copper carbonate does not melt and fully decompose until much higher. No wonder it is implicated in cases of glaze blistering and bubbling.
Links
| Glossary |
LOI
Loss on Ignition is a number that appears on the data sheets of ceramic materials. It refers to the amount of weight the material loses as it decomposes to release water vapor and various gases during firing. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
