| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Light Magnesium Carbonate
Alternate Names: Hydrated Magnesium Carbonate, Hydromagnesite, Magnesium Carbonate
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| MgO | 43.09% | 1.00 | |
| CO2 | 37.64% | n/a | |
| H2O | 19.26% | n/a | |
| Oxide Weight | 40.30 | ||
| Formula Weight | 93.50 | ||
Notes
A white very light-weight powder (and bag of this material is like a bag of feathers). It is practically insoluble in water. This is not the same material as magnesium carbonate (magnesite).
MagCarb is very refractory, it does not decompose and release its MgO to the glaze melt as readily as other sources. It is a good example of the need to consider mineralogy and material-level physics in addition to chemical makeup (when using glaze chemistry to adjust and fix glazes). Two glazes may have the same calculated chemistry, but the one using the MagCarb to source the MgO will not be melted as much.
MagCarb mattes glazes. MgO in sufficient percentages in glaze melts, is a classic matting agent. However, since magnesium carbonate is so refractory and does not readily release MgO, the matting mechanism is simply that it is refractory as a material and inhibits smooth-out of the melt (especially if added in larger percentages or employed at lower temperatures where making a good matte is more difficult).
Magnesium carbonate crawls glazes because it decreases melt mobility and the exceedingly small particle size of the material increases dry shrinkage (pulling the glaze into islands). MgO, in the portion that does disperse into the melt, has a high surface tension, which assists the melt in pulling itself into islands. Up to 35% can be found in some crawl recipes. The host glaze can be, on its own, highly melt fluid (e.g. very high in B2O3). A simple cone 10 recipe used by many is just 75:25 Nepheline:MagCarb (or 80:20).
MagCarb has a very high Loss on Ignition, this could cause glaze surface issues. Dolomite and talc more readily release their MgO to the glaze melt for a higher temperature glazes. There is conflicting information on the decomposition temperature, sources report figures that vary from 350C to 650C. Thus it is possible that a host glaze could be already fluid before this material has finished gassing, trapping it within to create the glass equivalent of an Aero chocolate bar. If you use a large percentage of this in a glaze (for crawling for example), it may be wise to halt the kiln at 650C and soak during the fire up. Or do a test firing to 700C, cool the kiln quickly and check the melted state of the glaze.
It is added (.12-.25%) to flocculate clay slurries, improving and stabilizing the set and suspension characteristics.
It is used as a electrolyte in both ground and cover coat enamels.
Light magnesium carbonate or hydromagnesite is made by precipitation from a boiled solution of magnesium sulfate and sodium carbonate.
pH (1% suspension): 9.5
Bulk Density: 130-140 g/l
Particle Size (+6 microns): 75% (+2 microns): 98%
Related Information
Snakeskin glazes are induced to crawl by this additive

This picture has its own page with more detail, click here to see it.
10% light magnesium carbonate has been added to this low-temperature terra cotta white glaze, G1916Q - it induces enough crawling to form islands and the MgO it sources also mattes the glaze. The bare areas between the islands have a glassy surface, thus the crawling begins at some point after the melt has begun to form an interfacial layer with the body. "Snakeskin" recipes like this effect can call for much higher percentages of magnesium carbonate (we have seen 30%) but such levels produce a much more matte surface and reduce thermal expansion so much that the islands could shiver (the G1916Q recipe is flexible to raise expansion if needed). Rather than bring a new recipe into your production (these recipes can be really weird, calling for unusual materials) it may be better to add just enough magnesium carbonate to your standard white gloss glaze. Even better, start with a fluid melt high expansion glaze (e.g. a crackle), it will provide more margin for thermal expansion reduction without shivering. Magnesium carbonate is highly refractory and works this way all the way to cone 10 (where higher percentages are likely required).
Ceramic materials can vary widely in density

This picture has its own page with more detail, click here to see it.
A bag of magnesium carbonate beside a bag of feldspar. Although the former weighs 25 kg (vs. 22.7 kg for the feldspar), clearly it is a dramatically lighter (per volume unit) material. Lifting that bag of Mag Carb feels like lifting a pillow!
Magnesium carbonate vs. oxide: One big difference

This picture has its own page with more detail, click here to see it.
Here is a screenshot of side-by-side recipes in my account at insight-live.com. It takes 120 mag carb to source the same amount of MgO as 50 mag ox. I just made the two recipes, went into calculation mode and kept bumping up the magcarb by 5 until the chemistry was the same. Note the LOI of the magcarb version is 40. This one would certainly crawl very badly.
LOI horse race with surprising winners
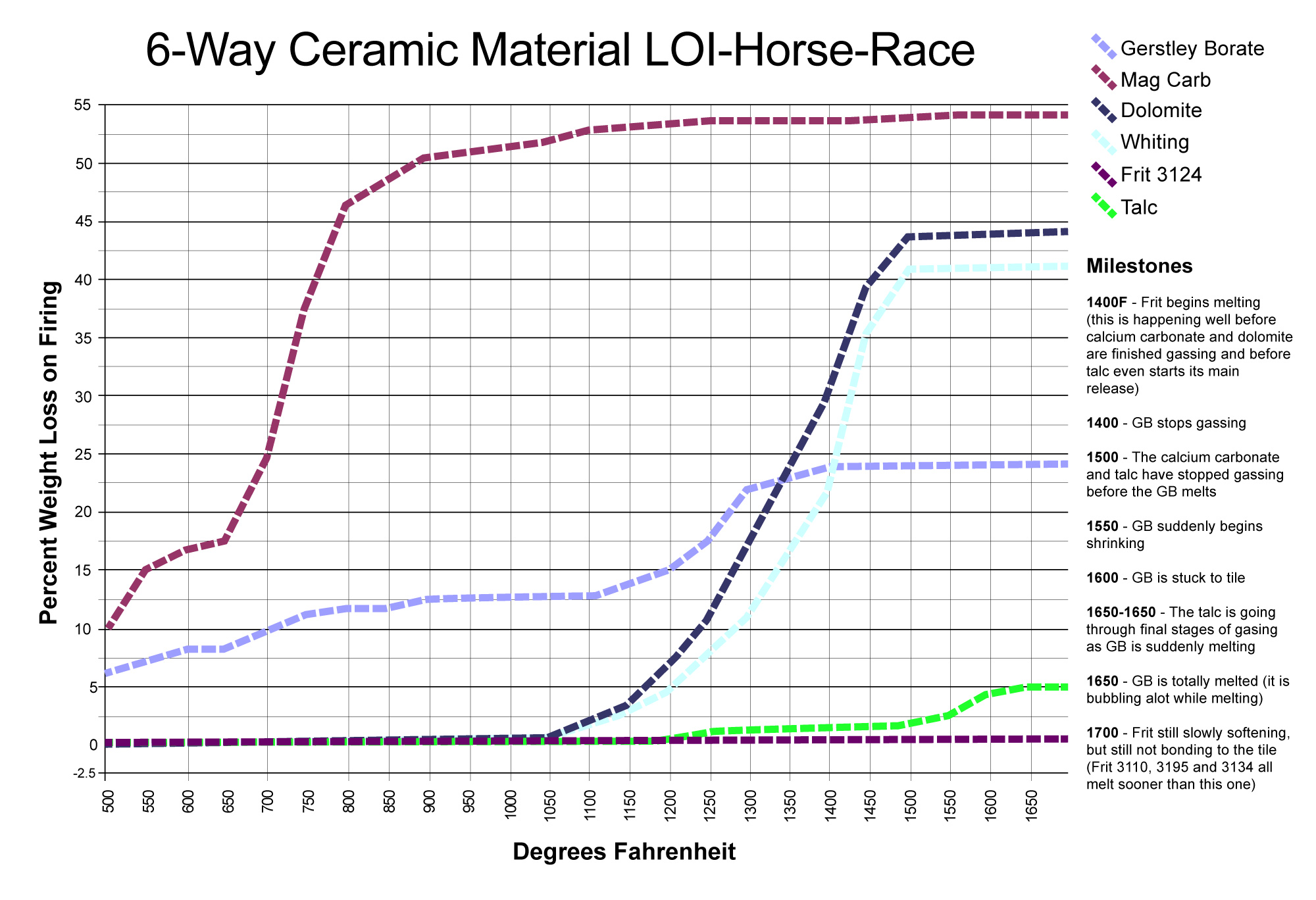
This picture has its own page with more detail, click here to see it.
This chart compares the decompositional off-gassing (% weight Loss on Ignition) behavior of six glaze materials as they are heated through the range 500-1700F. It is amazing how much weight some can lose on firing - for example, 100 grams of calcium carbonate generates 45 grams of CO2! This chart is a reminder that some late gassers overlap early melters. That is a problem. The LOI of these materials can affect glazes (causing bubbles, blisters, pinholes, crawling). Talc is an example: It is not finished gassing until 1650F, yet many fritted glazes have already begun melting by then. Even Gerstley Borate, a raw material, begins to melt while talc is barely finished gassing. Dolomite and calcium carbonate Other materials also create gases as they decompose during glaze melting (e.g. clays, carbonates, dioxides).
Mag carb causes crawling in higher amounts

This picture has its own page with more detail, click here to see it.
Example of two crawling glazes. Both have magnesium carbonate added to make this happen (around 10%). On the left at cone 04 on a terra cotta body, on the right at cone 6 on a porcelain. Magnesium carbonate also mattes glazes.
Links
| Materials |
Light Magnesium Carbonate C2FD
|
| Materials |
Talc
A source of MgO for ceramic glazes, a flux or thermal expansion additive in clay bodies, also used in the manufacture of cordierite. |
| Materials |
Magnesite
|
| Typecodes |
Generic Material
Generic materials are those with no brand name. Normally they are theoretical, the chemistry portrays what a specimen would be if it had no contamination. Generic materials are helpful in educational situations where students need to study material theory (later they graduate to dealing with real world materials). They are also helpful where the chemistry of an actual material is not known. Often the accuracy of calculations is sufficient using generic materials. |
| Typecodes |
Flux Source
Materials that source Na2O, K2O, Li2O, CaO, MgO and other fluxes but are not feldspars or frits. Remember that materials can be flux sources but also perform many other roles. For example, talc is a flux in high temperature glazes, but a matting agent in low temperatures ones. It can also be a flux, a filler and an expansion increaser in bodies. |
| URLs |
http://en.wikipedia.org/wiki/Hydromagnesite
Hydrated Magesium Carbonate at Wikipedia |
| URLs |
http://webmineral.com/data/Hydromagnesite.shtml
Hydromagnesite at webmineral.com |
| Oxides | MgO - Magnesium Oxide, Magnesia |
| Glossary |
Matte Glaze
Random material mixes that melt well overwhelmingly want to be glossy, creating a matte glaze that is also functional is not an easy task. |
| Troubles |
Crawling
Ask yourself the right questions to figure out the real cause of a glaze crawling issue. Deal with the problem, not the symptoms. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
