| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
All Temperature Numbers Temperature Listing
200-1000C (392-1832F)
Decarbonation
A wide range of carbonates are used in ceramics and each of these has its own thermal history. Within that is a range where they decompose to give off carbon dioxide or CO. Some of these materials lose alot of weight in the process (up to 50%). If decarbonation is still happening after glazes are starting to melt, glaze defects can be the result.
Related Information
Magnesium carbonate vs. oxide: One big difference

This picture has its own page with more detail, click here to see it.
Here is a screenshot of side-by-side recipes in my account at insight-live.com. It takes 120 mag carb to source the same amount of MgO as 50 mag ox. I just made the two recipes, went into calculation mode and kept bumping up the magcarb by 5 until the chemistry was the same. Note the LOI of the magcarb version is 40. This one would certainly crawl very badly.
LOI horse race with surprising winners
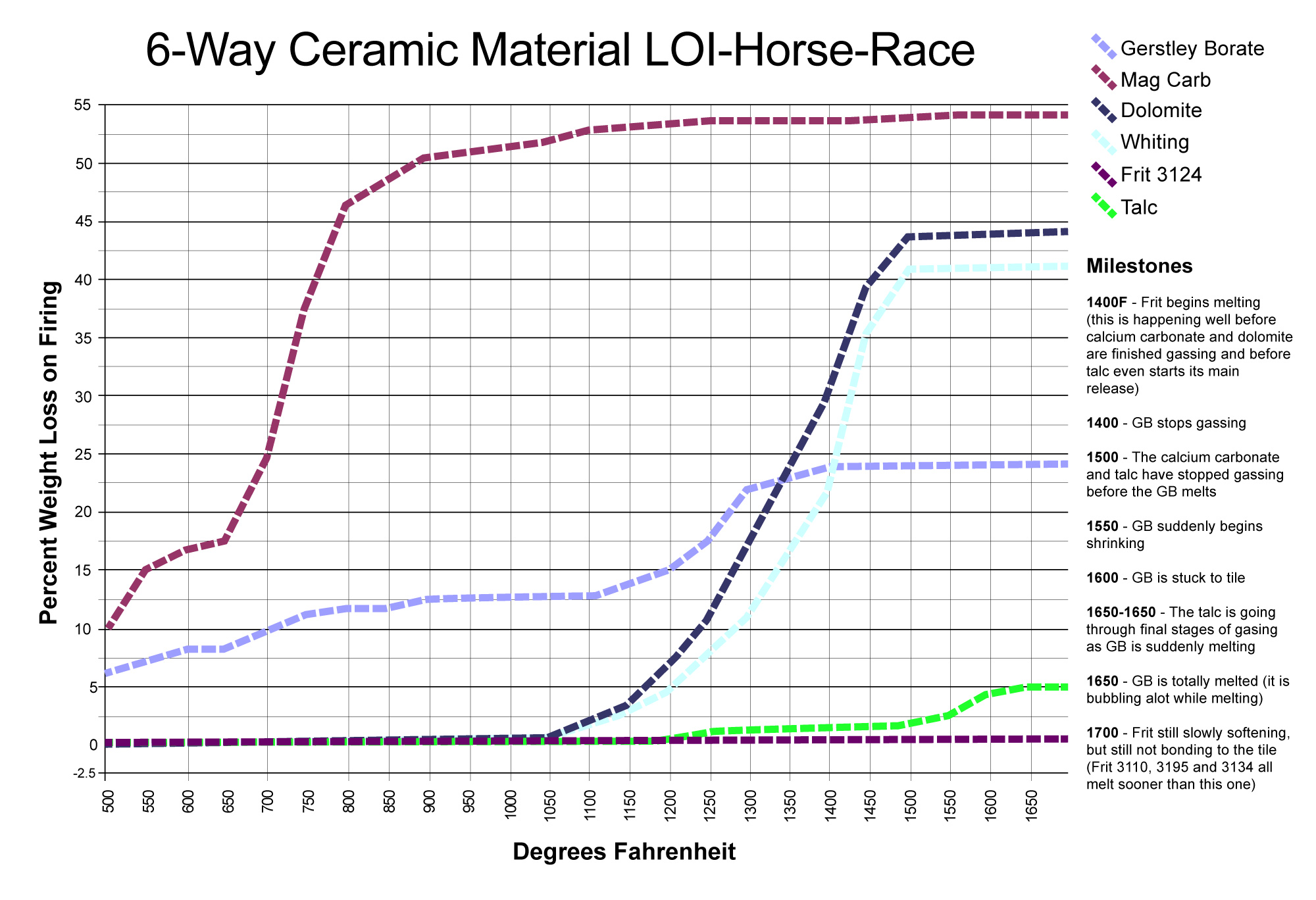
This picture has its own page with more detail, click here to see it.
This chart compares the decompositional off-gassing (% weight Loss on Ignition) behavior of six glaze materials as they are heated through the range 500-1700F. It is amazing how much weight some can lose on firing - for example, 100 grams of calcium carbonate generates 45 grams of CO2! This chart is a reminder that some late gassers overlap early melters. That is a problem. The LOI of these materials can affect glazes (causing bubbles, blisters, pinholes, crawling). Talc is an example: It is not finished gassing until 1650F, yet many fritted glazes have already begun melting by then. Even Gerstley Borate, a raw material, begins to melt while talc is barely finished gassing. Dolomite and calcium carbonate Other materials also create gases as they decompose during glaze melting (e.g. clays, carbonates, dioxides).
Micro bubbles in low fire glaze. Why?

This picture has its own page with more detail, click here to see it.
Left: G1916Q transparent fired at cone 03 over a black engobe (L3685T plus stain) and a kaolin-based low fire stoneware (L3685T). The micro-bubbles are proliferating when the glaze is too thick. Right: A commercial low fire transparent (two coats lower and 3 coats upper). A crystal clear glaze result is needed and it appears that the body is generating gases that cause this problem. Likely the kaolin is the guilty material, the recipe contains almost 50%. Kaolin has a 12% LOI. To cut this LOI it will be necessary to replace some or all of the kaolin with a low carbon ball clay. This will mean a loss in whiteness. Another solution would be diluting the kaolin with feldspar and adding more bentonite to make up for lost plasticity.
LOI test of common materials flags copper carbonate

This picture has its own page with more detail, click here to see it.
These are pure samples (with 2% binder added) of (top left to bottom right) strontium carbonate, nepheline syenite, cobalt carbonate, manganese dioxide, bentonite (in bowl), 6 Tile kaolin, New Zealand kaolin and copper carbonate. I am firing them at 50F increments from 1500F and weighing to calculate loss on ignition for each. I want to find out at what temperature they are gassing (and potentially bubble-disrupting the glaze they are in or under). Notice how the copper is fuming and spitting black specks on the shelf, this happens right around 1500F (these stains on the shelf darkened considerably when the kiln was fired higher). Yet copper carbonate does not melt and fully decompose until much higher. No wonder it is implicated in cases of glaze blistering and bubbling.
Calcium carbonate and dolomite are refractory when used pure

This picture has its own page with more detail, click here to see it.
Examples of calcium carbonate (top) and dolomite (both mixed with 25% bentonite to make them plastic enough to make a test bars). They are fired to cone 9. Both bars are porous and refractory, even powdery. However, put either of these in a mix with other ceramic minerals and they interact strongly to become fluxes.
Links
| Temperatures | Copper carbonate basic decomposes (300-330) |
| Temperatures | Copper carbonate basic breakdown (1050-) |
| Temperatures | Calcium carbonate decomposition (750-1000) |
| Temperatures | Strontium carbonate decomposition (800-1100) |
| Temperatures | Manganese Carbonate decomposes to MnO (200-) |
| Temperatures | Decomposition of Barium Carbonate (1025-) |
| Temperatures | Sodium Carbonate decomposes (850-) |
| Temperatures | Calcium carbonate, talc finished gassing (815-) |
| Glossary |
Carbon Burnout
Ceramic materials, especially clays, often contain carbon and organic compounds. When they are fired in a kiln, these must burn out, often producing complications. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
