| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Dolomite
Alternate Names: Calcium Magnesium Carbonate, Raw Limestone
Description: Double carbonate of magnesia/calcia
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 30.49% | 0.50 | |
| MgO | 21.90% | 0.50 | |
| CO2 | 47.61% | n/a | |
| Oxide Weight | 48.20 | ||
| Formula Weight | 92.00 | ||
Notes
Dolomite as a ceramic material is a uniform calcium magnesium carbonate. In ceramic glazes, it is used as a source of magnesia and calcia. Other than talc, dolomite is the principal source of MgO in high-temperature raw glazes. 'Dolomite matte' stoneware glazes, for example, are highly prized for their pleasant 'silky' surface texture. Dolomite by itself is refractory, but when combined with the typical oxides in a glaze (especially boron) it readily enters the melt.
Commercial dolomites are not able to achieve the theoretical 54:46 calcium carbonate:magnesium carbonate ratio, they tend to have less magnesia. It is simple to do an LOI test by firing a specimen of powder in a thin bisqued bowl to confirm the consistency of dolomite shipments. The chemistry shown here is theoretical and many commercial materials approach this with much less than 1% of two or three other oxides (e.g. Al2O3, SiO2).
Dolomite is a carbonate (like whiting) in that it loses considerable weight during firing when it disassociates to form MgO, CaO and CO2, this process being complete by about 900C. That being said, dolomite gases out before talc does so for boron glazes that begin melting early, sourcing MgO from dolomite will produce a smoother and more defect-free surface (because of the comparative absence of tiny bubbles in the glass matrix).
In many circumstances where a raw glaze employs both CaO and MgO, dolomite is an economic alternative to sourcing with a mix of calcium carbonate and talc. However, care needs to be taken to obtain a consistent grade since dolomites tend to vary more in mineralogy and can contain iron contamination that can darken the fired glaze. Although calcium carbonate and dolomite are plentiful minerals and grinding plants are located throughout North America, finding a suitable ceramic-grade dolomite that will be consistent and available long term is not as easy as it might seem.
Synthetic substitutes to source MgO and CaO (e.g. frits) are worth considering, especially if glazes are not high temperature. Frits have no loss on ignition (therefore do not generate glaze bubbles) and melt far earlier than mineral sources of MgO and CaO. Using glaze chemistry it is quite easy to adjust a recipe to source MgO from a frit instead of raw materials.
Related Information
LOI horse race with surprising winners
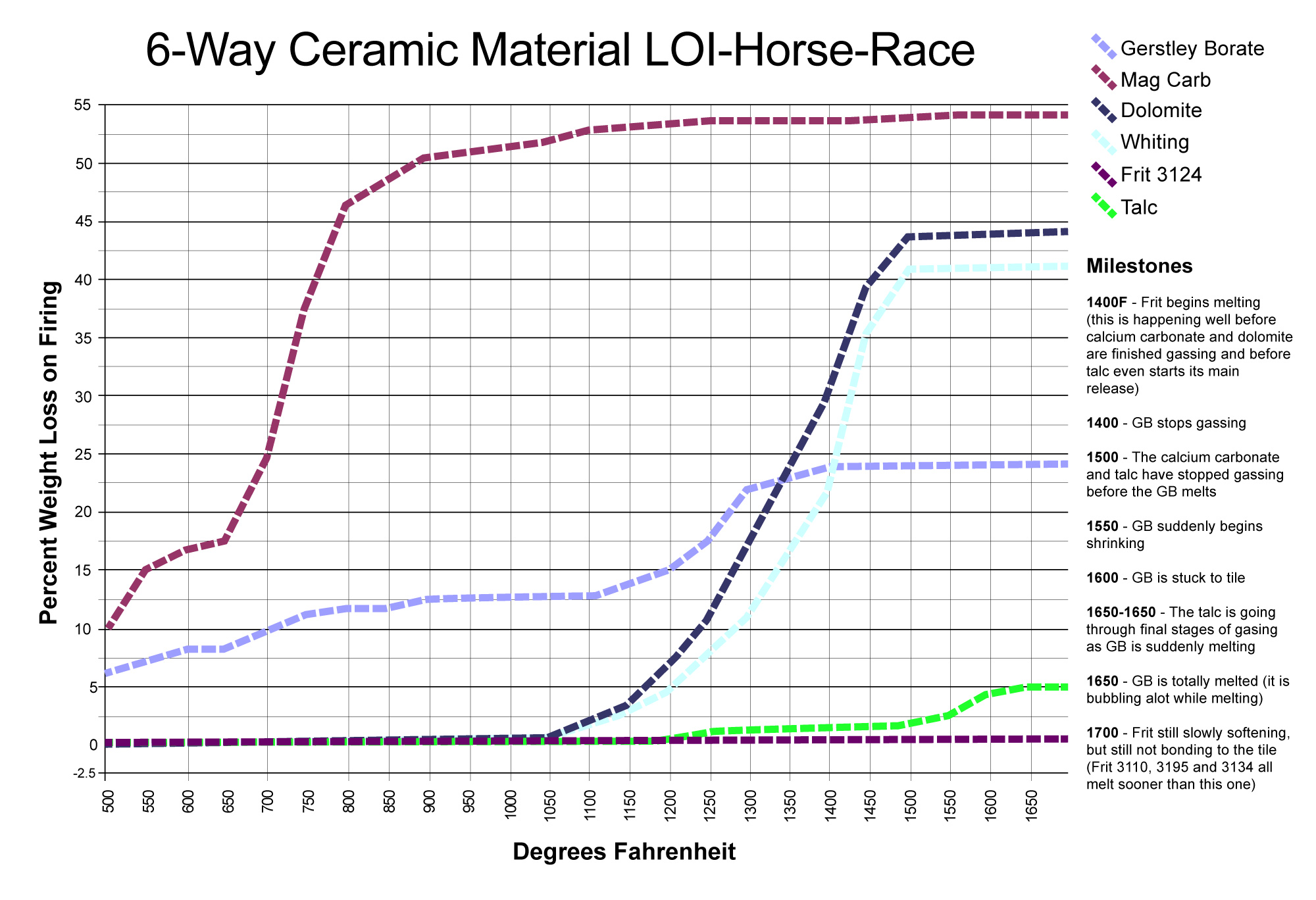
This picture has its own page with more detail, click here to see it.
This chart compares the decompositional off-gassing (% weight Loss on Ignition) behavior of six glaze materials as they are heated through the range 500-1700F. It is amazing how much weight some can lose on firing - for example, 100 grams of calcium carbonate generates 45 grams of CO2! This chart is a reminder that some late gassers overlap early melters. That is a problem. The LOI of these materials can affect glazes (causing bubbles, blisters, pinholes, crawling). Talc is an example: It is not finished gassing until 1650F, yet many fritted glazes have already begun melting by then. Even Gerstley Borate, a raw material, begins to melt while talc is barely finished gassing. Dolomite and calcium carbonate Other materials also create gases as they decompose during glaze melting (e.g. clays, carbonates, dioxides).
Dolomite Crystals

This picture has its own page with more detail, click here to see it.
Testing a new brand of dolomite

This picture has its own page with more detail, click here to see it.
Dolomite is a key material for glazes, especially mattes. We were forced to adopt a new brand and needed confidence it was equivalent. Three tests were done to compare the old long-time-use material (IMASCO Sirdar) with a new one (LHoist Dolowhite). The first melt flow tester compares them in a very high dolomite cone 6 recipe formulated for this purpose; the new material runs just slightly more. The second tester is uses the G2934 cone 6 magnesia matte recipe with 5% black stain; the new material runs a little less here. The third test is the high dolomite glaze on a dark burning clay to see the translucency and compare the surface character. They are very close. These three gave us the confidence to proceed.
When both mineralogy and chemistry are shown on a data sheet

This picture has its own page with more detail, click here to see it.
Some material data sheets show both the oxide and mineralogical analyses. Dolomite, for example, is composed of calcium carbonate and magnesium carbonate minerals, these can be separated mechanically. Although this material participates in the glaze melt to source the MgO and CaO (which are oxides), it's mineralogy (the calcium and magnesium carbonates) specifically accounts for the unique way it decomposes and melts.
The difference between dolomite and calcium carbonate in a glaze

This picture has its own page with more detail, click here to see it.
These glaze cones are fired at cone 6 (without slow cooling) and have the same recipe: 20 Frit 3134, 21 EP Kaolin, 27 calcium carbonate, 32 silica. The difference: The one on the left uses dolomite instead of calcium carbonate. Notice how the MgO from the dolomite mattes the surface (without slow cooling) whereas the CaO from the calcium carbonate produces a brilliant gloss (because it needs slow cooling).
Calcium carbonate and dolomite are refractory when used pure

This picture has its own page with more detail, click here to see it.
Examples of calcium carbonate (top) and dolomite (both mixed with 25% bentonite to make them plastic enough to make a test bars). They are fired to cone 9. Both bars are porous and refractory, even powdery. However, put either of these in a mix with other ceramic minerals and they interact strongly to become fluxes.
2, 5, 10, 15% dolomite added to Ravenscrag Slip at cone 10R

This picture has its own page with more detail, click here to see it.
This is a buff stoneware clay. Crystal development toward a dolomite matte begins at 15%. By Kat Valenzuela.
Ravenscrag Slip based dolomite matte

This picture has its own page with more detail, click here to see it.
GR10-J Ravenscrag dolomite matte base glaze at cone 10R on Plainsman H443 iron speckled clay (actually, the MgO is being sourced from talc instead of dolomite). This recipe was created by starting with the popular G2571 base recipe (googleable) and calculating a mix of materials having the maximum possible Ravenscrag Slip percentage. The resultant glaze has the same excellent surface properties (resistance to staining and cutlery marking) but has even better application and working properties. It is a little more tan in color because of the iron content of Ravenscrag Slip.
Does iron oxide stain a dolomite body red? Nope!

This picture has its own page with more detail, click here to see it.
These fired bars are the L4410P low temperature clay body (it replaces the traditional 50% talc with 40% dolomite and 10% nepheline). These bars are fired from cone 5 down to cone 06 (top to bottom). The body contains 4% red iron oxide, this would normally be enough to produce a bright red fired color. But clearly, the dolomite is killing its development. A better option is to use the L4170 plastic terra cotta (or its L4170B casting version).
Why this dolomite body bisqueware is splitting after sitting around

This picture has its own page with more detail, click here to see it.
On the loss of talc earlier this year we had to reformulate a low fire white burning body to use dolomite instead, recipe L4410P (like talc it raises thermal expansion to ensure fit of commercial glazes). As its advantages and disadvantages become evident we have been documenting them on the Snow page. A recent revelation has been the matter of rehydration of the limestone (dolomite is ground limestone): Bisque firing dehydrates it. The dolomite particles are neutralized somewhat by being isolated and having reacted to some extent with neighbouring clay and feldspar particles. Further, during dehydration, they leave considerable porosity into which they should be able to reexpand later if needed. This photo demonstrates something we have not seen in our dry climate: These 3D-printed bisque pieces have spontaneously cracked after sitting around for some time in the much damper climate of southern Ontario. In some cases, swelling occurs around the cracks. Until we can further tune the recipe to chemically tie up the dolomite take some precautions when using this type of body. Glaze ware soon after bisquing. Dry it as quickly as possible after glazing. If any surface has not been glazed then render it impervious to water penetration by using a silicone sealer. Photo courtesy of Nilou Ghaemi, Sheridan College.
Are dolomite clay bodies OK?

This picture has its own page with more detail, click here to see it.
The L4410P cone 04 body (Plainsman Snow), although high in Dolomite has proven to be very resilient, but not just in physical strength. This was developed as an alternative to talc bodies. This tile has not spalled despite cycling it three times through an hours-long water immersion then a week-long freezer stay. The mug has cracked after four years of storage on a shelf in our lab (but it has 60% dolomite vs. Snow's 40%). If the percentage of dolomite (or calcium carbonate) is too high, cracks like this can develop as the dolomite hydrates. For use in pottery, these can be avoided by treating pieces with a silicone sealant.
Four cone 04 glazes cover a brown engobe and dolomite body differently

This picture has its own page with more detail, click here to see it.
These are Plainsman Snow (the L4410P recipe using dolomite) cast pieces with L3685Z7 stained engobe on the upper sections. The four sides have been clear glazed with Mayco S-2101, Duncan PB001, Amaco LG-10 and Spectrum 700 transparent glazes. The left half of each has an extra glaze layer, that has not made much difference in any of them. The S-2101 transmits the brown color best, the LG-10 and PB001 a little less. The 700 is decidedly lighter, it is altering the brown color (likely MgO in the glaze is reacting with the stain). However, over the white body, the 700 is by far the best, being crystal clear and glassy smooth. The lower halves of these also demonstrate something equally interesting: The ones that don't alter the brown do react with the MgO in the white body, yellowing it and turning opaque in areas. The lesson is to try various brands of clear glazes over underglaze decoration and colored engobes, especially with dolomite-based low fire bodies. It might even be advantageous to use one brand on the insides of pieces and another on the engobe or slip-decorated exteriors.
A body so porous that it has absorbed its own glaze!

This picture has its own page with more detail, click here to see it.
This unbelievable body is made from dolomite, 65% of it. There is 35% ball clay to give it workability and 5% Ferro frit 3110. The frit stabilizes it so that the fired body does not rehydrate. This has a porosity of 35%! And that porosity is stable across the entire range we have fired it at (cone 06-6). The single-layer on the lower portion has been completely absorbed, the double-layer on the upper is almost gone.
How to make a zero fired shrinkage clay

This picture has its own page with more detail, click here to see it.
This is Plainsman BGP, a terra cotta, mixed with 30% dolomite. Note the "DSHR" column in the SHAB test data (third last column): The drying shrinkage still averages over 7% even with the 30% dolomite, so BGP is very plastic. Notice the "FSHR" (fired shrinkage) column, it is negative for the first five test bars fired at cone 05-01, which means the bars grew in size! But notice the shrinkage hits 0% at cone 1 (bar #6). By cone 2 the trend has reversed to 0.3% shrinkage. The #6 bar is appears to be vitrifying, the color is darkening and it is strong. But notice the last "ABS" column (water absorption), it is 18.7%! This body was intended as a high-porosity ceramic at the lower ranges (it has 25% porosity at cone 05), but the dolomite is also slowing the densification as it goes through the vitrification process. Without the dolomite the top bar would be melting! By cone 1 its firing shrinkage would be 7%, and the porosity would be zero. Is this technique practical? Yup! The entire monoporosa wall tile industry is based on it!
QRCode mounted on Plainsman "Snow" tile

This picture has its own page with more detail, click here to see it.
Plainsman Snow clay (developed under code number L4410P) makes this QRCode mosaic possible. Each 8mm square porcelain pixel is glaze-glued onto a 21cm square 5mm thick ceramic tile made of Snow (simply rolled and cut and dewatered between sheets of Gyproc). During the bisque firing to cone 04, the Snow tile had zero shrinkage, from dry to fired, and therefore did not curl up at the edges. On refiring to melt the glaze it again stays flat. No other common plastic clay can do this! Snow continues this zero-shrinkage performance for seven more cones of firing (all the way to 4). The secret is the 40% dolomite it contains. We silicone-sealed this work front and back, now it is ready for outdoor installation.
Links
| Materials |
Talc
A source of MgO for ceramic glazes, a flux or thermal expansion additive in clay bodies, also used in the manufacture of cordierite. |
| Materials |
Limestone
|
| Materials |
Camadil 95 Dolomite
|
| Materials |
Calcined Dolomite
|
| Materials |
Dolocron 40-13
|
| Materials |
Kuncice Dolomite
|
| Materials |
Dolowhite
|
| Materials |
KARIBIB Dolomite
|
| Materials |
Magnesite
|
| Typecodes |
Generic Material
Generic materials are those with no brand name. Normally they are theoretical, the chemistry portrays what a specimen would be if it had no contamination. Generic materials are helpful in educational situations where students need to study material theory (later they graduate to dealing with real world materials). They are also helpful where the chemistry of an actual material is not known. Often the accuracy of calculations is sufficient using generic materials. |
| Typecodes |
Flux Source
Materials that source Na2O, K2O, Li2O, CaO, MgO and other fluxes but are not feldspars or frits. Remember that materials can be flux sources but also perform many other roles. For example, talc is a flux in high temperature glazes, but a matting agent in low temperatures ones. It can also be a flux, a filler and an expansion increaser in bodies. |
| Oxides | CaO - Calcium Oxide, Calcia |
| Oxides | MgO - Magnesium Oxide, Magnesia |
| Glossary |
Matte Glaze
Random material mixes that melt well overwhelmingly want to be glossy, creating a matte glaze that is also functional is not an easy task. |
| Minerals |
Dolomite
Dolomite, as a rock, is called "dolomitic limestone". It is a carbonate, similar to limestone, but h |
| URLs |
http://www.mineralszone.com/minerals/dolomite.html
Dolomite at mineralszone.com |
| URLs |
https://en.wikipedia.org/wiki/Dolomite_(rock)
Dolomite rock at Wikipedia |
Mechanisms
| Glaze Matteness | Dolomite can be used in glazes melting over 1170C to produce a silky matte surface. This occurs because high percentages of dolomite help to form diopside crystals (CaMg(SiO3)2) on cooling, and it is these that produce the popular butter-matte effect. This effect is most pronounced in reduction. |
|---|
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
