| Monthly Tech-Tip | No tracking! No ads! |
All Temperature Numbers Temperature Listing
760C (1400F)
Common frits begin melting
Common boron frits begin melting before talc, dolomite and calcium carbonate have completed gassing. A glaze containing 20% Ferro Frit 3134, for example, could reach full density (zero porosity) by less than 1800F (typical bisque temperature). Such would acts as a barrier to the escape of gases.
Related Information
Melt fluidity comparison of frits - 1400F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1400F and held for 15 minutes. Frit 3134 is still expanding. 3602 is also starting to flow. A number of them are shrinking and densifying like a porcelain would.
A frit softens over a wide temperature range

This picture has its own page with more detail, click here to see it.
Raw materials often have a specific melting temperature (or they melt quickly over a narrow temperature range). We can use the GLFL test to demonstrate the development of melt fluidity between a frit and a raw material. On the left we see five flows of boron Ferro Frit 3195, across 200 degrees F. Its melting pattern is slow and continuous: It starts flowing at 1550F (although it began to turn to a glass at 1500F) and is falling off the bottom of the runway by 1750F. The Gerstley Borate (GB), on the other hand, goes from no melting at 1600F to flowing off the bottom by 1625F! But GB has a complex melting pattern, there is more to its story. Notice the flow at 1625F is not transparent, that is because the Ulexite mineral within GB has melted but its Colemanite has not. Later, at 1700F, the Colemanite melts and the glass becomes transparent. Technicians call this melting behaviour "phase transition", that does not happen with the frit.
These common Ferro frits have distinct uses in traditional ceramics

This picture has its own page with more detail, click here to see it.
I used Veegum to form 10 gram GBMF test balls and fired them at cone 08 (1700F). Frits melt really well, they do have an LOI like raw materials. These contain boron (B2O3), it is a low expansion super-melter that raw materials don’t have. Frit 3124 (glossy) and 3195 (silky matte) are balanced-chemistry bases (just add 10-15% kaolin for a cone 04 glaze, or more silica+kaolin to go higher). Consider Frit 3110 a man-made low-Al2O3 super feldspar. Its high-sodium makes it high thermal expansion. It works really well in bodies and is great to make glazes that craze. The high-MgO Frit 3249 (made for the abrasives industry) has a very-low expansion, it is great for fixing crazing glazes. Frit 3134 is similar to 3124 but without Al2O3. Use it where the glaze does not need more Al2O3 (e.g. already has enough clay). It is no accident that these are used by potters in North America, they complement each other well (equivalents are made around the world by others). The Gerstley Borate is a natural source of boron (with issues frits do not have).
Cone 6 glazes can seal the surface surprisingly early - melt flow balls reveal it

This picture has its own page with more detail, click here to see it.
These are 10 gram balls of four different common cone 6 clear glazes fired to 1800F (bisque temperature). How dense are they? I measured the porosity (by weighing, soaking, weighing again): G2934 cone 6 matte - 21%. G2926B cone 6 glossy - 0%. G2916F cone 6 glossy - 8%. G1215U cone 6 low expansion glossy - 2%. The implications: G2926B is already sealing the surface at 1800F. If the gases of decomposing organics in the body have not been fully expelled, how are they going to get through it? Pressure will build and as soon as the glaze is fluid enough, they will enter it en masse. Or, they will concentrate at discontinuities and defects in the surface and create pinholes and blisters. Clearly, ware needs to be bisque fired higher than 1800F.
LOI horse race with surprising winners
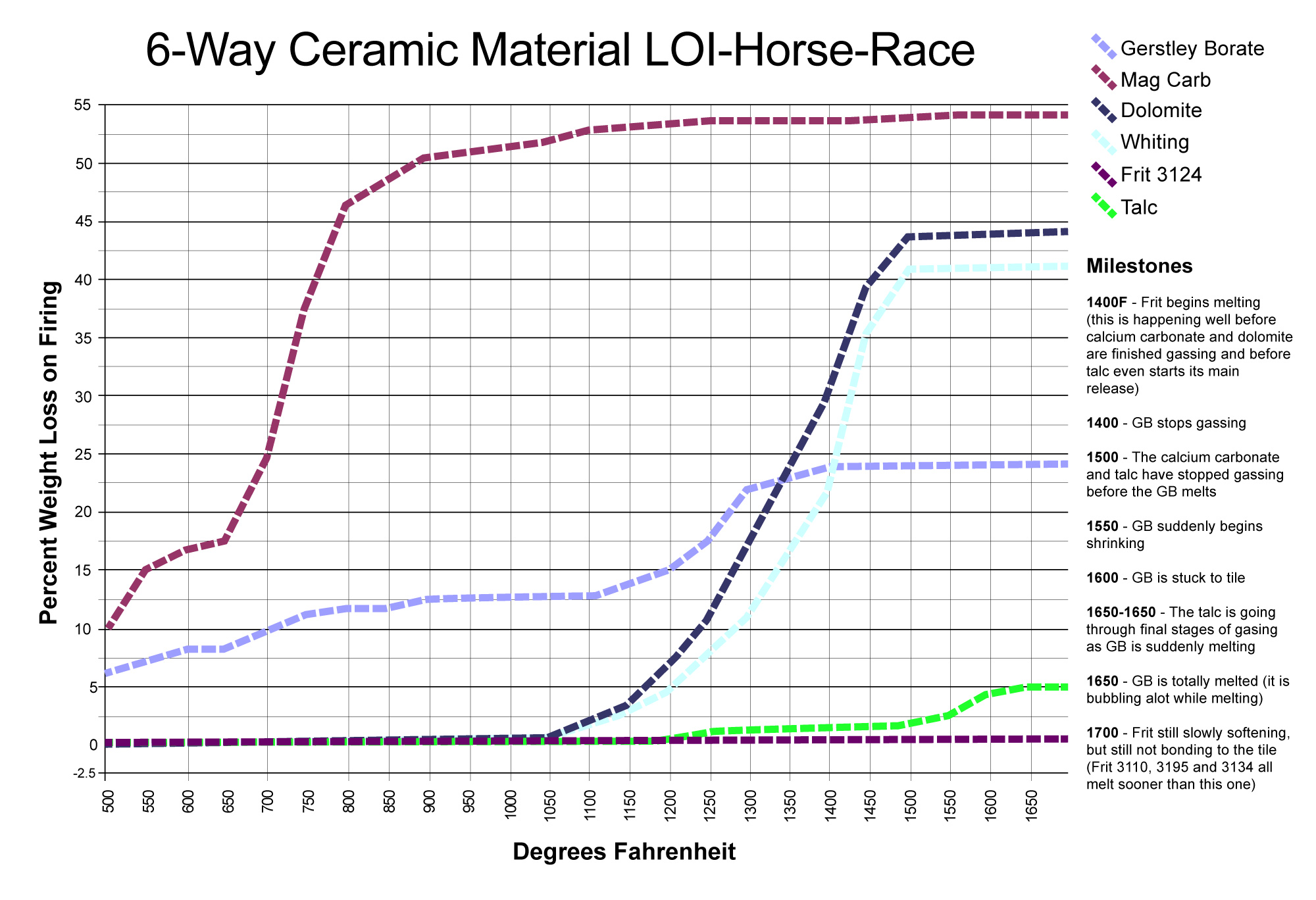
This picture has its own page with more detail, click here to see it.
This chart compares the decompositional off-gassing (% weight Loss on Ignition) behavior of six glaze materials as they are heated through the range 500-1700F. It is amazing how much weight some can lose on firing - for example, 100 grams of calcium carbonate generates 45 grams of CO2! This chart is a reminder that some late gassers overlap early melters. That is a problem. The LOI of these materials can affect glazes (causing bubbles, blisters, pinholes, crawling). Talc is an example: It is not finished gassing until 1650F, yet many fritted glazes have already begun melting by then. Even Gerstley Borate, a raw material, begins to melt while talc is barely finished gassing. Dolomite and calcium carbonate Other materials also create gases as they decompose during glaze melting (e.g. clays, carbonates, dioxides).
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
