| Monthly Tech-Tip | No tracking! No ads! |
High B2O3 imparts better melt fluidity, but also fewer micro-bubbles
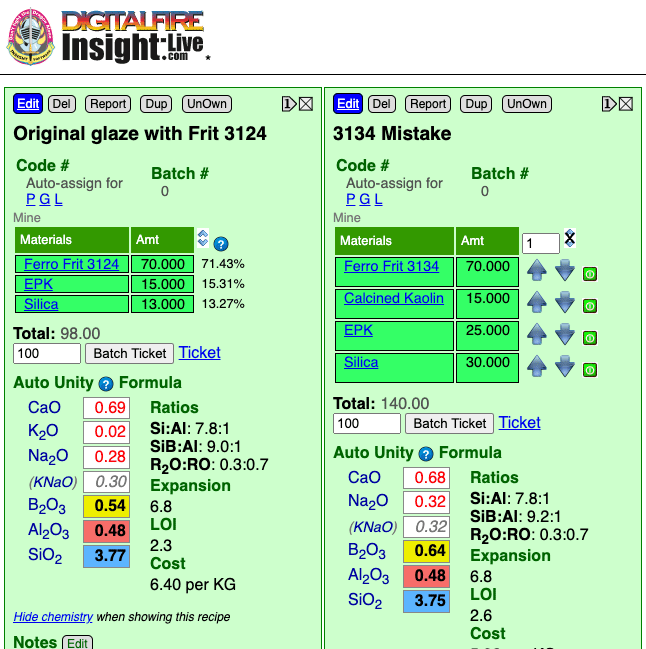
A cone 6 firing. The glaze on the left has a B2O3 molar content of 0.54 whereas the one on the right has 0.64 (other oxide levels are the same). This is triple the typical amount of boron in a cone 6 glaze, the result is obvious: High melt fluidity for both. But G3904A has a significant difference: The flow is more transparent because of the lower micro-bubble population. It's melt better enables the bubbles to pass, exit and the surface to heal. Why don't all glazes use more boron? Cost. Frits are expensive and they are the best source of boron. There is also a cost to durability (although mitigated when there is plenty of Al2O3 and SiO2 present, as is the case here). These recipes were part of a project to fix a recipe where the potter mistakenly used Frit 3134 instead of 3124 when mixing a large batch of glaze. I calculated how much kaolin and silica to add to bring the chemistry back into line with the original. This was possible because frit 3134 chemistry is an approximate oxide-subset of 3124. The resultant glaze is potentially better than the original.
Related Pictures
A large glaze batch mixing error rescued using glaze chemistry
This picture has its own page with more detail, click here to see it.
The person used Frit 3134 instead of 3124, it makes up 70% of the recipe. The glaze melted much more and ran off the ware. That sounds like an impossible-to-fix problem. It just so happens that these frits have very similar chemistry except for one thing: 3134 has almost no Al2O3. That means that kaolin can be added to the bad batch to source the missing Al2O3 (and replenish the shortage of SiO2 at the same time). Extra silica is also needed to restore the full SiO2. The new chemistry is not an exact match, the B2O3 is a little higher, but this should not be an issue. Of course a raw:calcine mix of kaolin is needed (to prevent the glaze from shrinking too much on drying and therefore cracking). From this calculation we can see that for every 100 grams of the original powder we need to add 10 EPK, 15 calcined kaolin and 17 silica. Of course, one would need to know the water content of the slurry, that is calculated as (weight wet - dry weight)/wet weight * 100. If the slurry was 50% water, for example, then every 200 grams of slurry would contain 100 grams of powder.
Videos
Links
| URLs |
https://insight-live.com/insight/share.php?z=682QPmFGJ3
Fixing a glaze mistakenly made using frit 3134 instead of 3124 |
| Materials |
Ferro Frit 3124
A commonly available calcium borosilicate frit. |
| Oxides | B2O3 - Boric Oxide |
| Glossary |
Boron Frit
Most ceramic glazes contain B2O3 as the main melter. This oxide is supplied by great variety of frits, thousands of which are available around the world. |
| Glossary |
Melt Fluidity
Ceramic glazes melt and flow according to their chemistry, particle size and mineralogy. Observing and measuring the nature and amount of flow is important in understanding them. |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy

