| Monthly Tech-Tip | No tracking! No ads! |
The secret of the higher gloss glaze on the right? A lead frit addition.
These cone 04 glazes have the same recipe (a version of Worthington Clear sourcing B2O3 from Ulexite instead of Gerstley borate). But the one on the right is more glassy, more transparent. Why? It has 10% added lead bisilicate frit. Lead bisilicate produces dazzling transparent glazes. no other method matches it. While potters gasp at the thought of using lead consider this: They thrive on unstable flux-deprived, glass-deprived and alumina-deprived base stoneware glazes with additions of large percentages of toxic colorants like chrome and manganese!
Related Pictures
UK Slipware: A Tradition of Terra Cotta and Lead Glaze
No borosilicate glaze can do this

This picture has its own page with more detail, click here to see it.
The traditional UK slipware is possible because of the brilliant gloss and hyper transparency of glaze made using lead bisilicate frits. The lead glaze interacts with the colors in underlying slips, dissolving and feathering them (as enabled by the time and temperature of the kiln). Interactions with iron produce warm colors. Ware is bisque fired after the decorating and drying (lower left), then dipped in the leaded glaze. Photo courtesy of Russell Kingston, Lynmouth Slipware Pottery.
Lead bisilicate frit data sheet claims high resistance to leaching
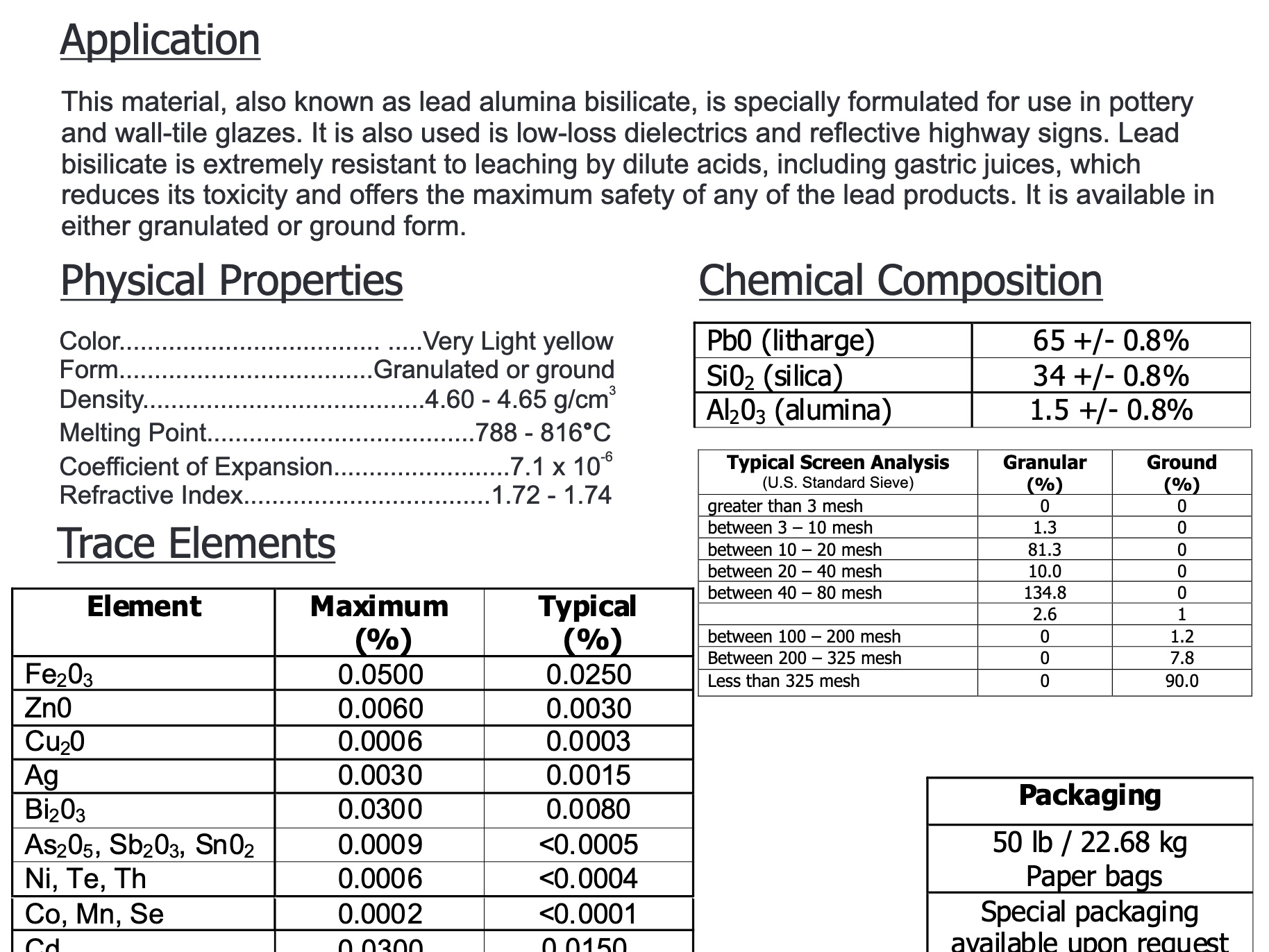
This picture has its own page with more detail, click here to see it.
Notice it is specifically "formulated for use in pottery glazes". And that "Lead bisilicate is extremely resistant to leaching by dilute acids, including gastric juices, which reduces its toxicity and offers the maximum safety of any of the lead products." In many countries, the use of lead glazes is still considered normal and safe. There is zero use of lead in pottery glazes in North America. Is it possible that we have tarred all lead products with the same brush? Could we at least be using it on non-functional decorated surfaces of low-fire stoneware? This would drastically reduce the energy consumption of kilns.
Videos
Links
| URLs |
https://insight-live.com/insight/share.php?z=9TkiB9GPkE
Insight-Live.com share for Worthington Clear with Lead |
| Glossary |
Lead in Ceramic Glazes
Lead is a melter in ceramic glazes and performs exceptionally well and must be misused to be toxic. It is also now environmentally pervasive. It is toxic and cumulative at any level of exposure. |
| Materials |
Lead Bisilicate Frit
A standard frit of 1 molar part of PbO and 2 of SiO2. It is considered stable and non-leachable. |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy

