Search Results - Pictures
-
Tap picture for full size and resolution
 A fireclay that is not really a fireclay!
A fireclay that is not really a fireclay!
This is a Lincoln 60 fireclay drying disk (that has been fired to cone 10R). It has near zero-porosity and is dense and very strong. It is like a stoneware clay, quite vitreous. …
-
Tap picture for full size and resolution
 A red fireclay from cone 7 (bottom) to cone 10 and 10R
A red fireclay from cone 7 (bottom) to cone 10 and 10R
Notice how much the color changes as the clay fires to greater maturity. This is Plainsman FireRed. …
-
Tap picture for full size and resolution
 Different runs of Plainsman Red Fireclay
Different runs of Plainsman Red Fireclay
These are Fire-Red, a red refractory low plasticity clay mined in Manitoba, Canada. Red fireclay are not common. The top bar on each set is fired to cone 10R, the iron imparts deep color but the matrix is still fairly pours. The next one down in each set is fired to cone 10 oxidation. The third one down is cone 8, the red color is holding (it shifts to brown between around cone 9). …
-
Tap picture for full size and resolution
 Hawthorne fireclay is really more of a stoneware clay
Hawthorne fireclay is really more of a stoneware clay
This is test-result data as reported from an account at Insight-live.com from a test done in 2002. The numbers in the first column in the shrinkage absorption section are the specimen numbers, they bars are labelled (on the right) with them. This clay is already quite dense and vitreous by cone 10 oxidation and reduction (having a 3% porosity) and it has a high fired shrinkage (more than 8%). Drying shrinkage is very low and its drying factor (performance) is good (even though it is quite plastic). The manufacturers data sheet shows higher firing shrinkage figures, however their numbers are actually total fired+drying shrinkage. …
-
Tap picture for full size and resolution
 How can we tell if a clay is really a fireclay without doing a PCE?
How can we tell if a clay is really a fireclay without doing a PCE?
While a PCE test is certainly desirable, few people or companies have the ability to do this test. But the more accessable SHAB test produces physical testing data that is perhaps even better. The last column of numbers (titled "ABS", for absorption) prove that the clay on the right, my code number L4380, is very refractory. Even at cone 10R it has 11.3% absorption, and its firing shrinkage is only 3.9%. How do I know cone 10 11.3% porosity is impressive for a fireclay? Because I have tested many other fireclays using this same procedure. What about the clay on the left, L4378? In comparison, its cone 10R porosity of 6.4%, making it quite a bit more vitreous. But not enough to qualify it as a stoneware (which would have 1-2% absorption and 6-7% firing shrinkage). Additionally, many common fireclays also exhibit same level of maturity as L4378. So is it a fireclay or a stoneware? A 10% feldspar addition would convert it to stoneware, so there is merit in calling it a "stoneware material". But that is "in comparison to" the very refractory L4380. But, if we were compare L4378 to any of the false fireclays commonly sold, then it could certainly be termed a fireclay. …
-
Tap picture for full size and resolution
 IMCO 400 Fireclay fired bars
IMCO 400 Fireclay fired bars
Cone 10R top, 11 oxidation and downward below that. This material, although called a "fireclay", is more fine grained and much more vitreous than what would normally be considered a refractory fireclay. Is it similar to Lincoln Fireclay (from California). …
-
Tap picture for full size and resolution
 IMCO 800 Fireclay fired bars
IMCO 800 Fireclay fired bars
Cone 10R (bottom) and cone 10, 8, 7 and 6 oxidation. …
-
Tap picture for full size and resolution
 IMCO C-Red: An exceptional red fireclay
IMCO C-Red: An exceptional red fireclay
These test bars are fired from cone 10 reduction (top) and 10 oxidation down to 6 oxidation. The drying shrinkage and the fired porosity and shrinkage are shown on the chart. This is a refractory clay, even at cone 10. It has excellent plasticity, similar to a typical throwing pottery clay. This can do at cone 10 what Redart does at low fire: Be added to a white burning clay body and turn it red. But 75% or more Redart is needed while as little as 25% if this can make a body red. While Redart is not plastic enough to use alone, this is (so it will have minimal impact on the drying and plasticity of the body). Since C-Red is refractory it will reduce the maturity of vitreous cone 6 bodies. But that is good because achieving red coloration at stoneware temperatures depends on a body not being vitreous (vitrification turns the color to brown). We tried adding only 30% of this to M370 porcelain, that produced a deep red stoneware. …
-
Tap picture for full size and resolution
 Is Lincoln 60 really a fireclay? Simple physical testing says...
Is Lincoln 60 really a fireclay? Simple physical testing says...
Materials are not always what their name suggests. These are Lincoln #60 Fireclay test bars fired at cone 10 reduction (top) and from cone 11 down to 6 oxidation (top to bottom). This clay already has stoneware density at cone 7 (3% porosity as indicated by our SHAB test). It vitrifies progressively from there upward (less than 3% porosity at cone 7 to near zero% by cone 9 oxidation. Maximum firing shrinkage happens at cone 8 and by cone 10 it is expanding (indicating decomposition has started) and it is bloating by cone 11 (melting is sealing the escape of gases of decomposition). Is Lincoln #60 a really fireclay? Absolutely not! But at cone 6 it is a credible plastic stoneware, all by itself! …
-
Tap picture for full size and resolution
 Jordan Fireclay fired test bars
Jordan Fireclay fired test bars
Cone 6 to 10 oxidation (top to bottom) fired shrinkage and porosity testing bars. …
-
Tap picture for full size and resolution
 Laguna B-Mix, B-Mix+Fireclay with Ravenscrag GR10-A, GR10-C glazes
Laguna B-Mix, B-Mix+Fireclay with Ravenscrag GR10-A, GR10-C glazes
Left two mugs are glazed with pure Ravenscrag Slip (roast:raw combo), far right one is RavenTalc silky matte (GR10-C). The speckled mugs have 10% of a Plainsman Fire-Red (a blend of a red fireclay, M2 and a heavily speckled ball clay). Ravenscrag Slip is an ideal base for cone 10R glazes, so many glazes can be made by adding pigments, opacifiers, variegators and matting agents. …
-
Tap picture for full size and resolution
 Lincoln Fireclay is plastic, it can be thrown on a potters wheel
Lincoln Fireclay is plastic, it can be thrown on a potters wheel
The left two were made from a 100% mix of Lincoln 60, the right one adds 2% bentonite (Lincoln 60, by itself, matures into a stoneware at around cone 8). While it was possible to throw the pure material, the plasticity is a little lower than a normal pottery clay. That being said, it is smooth and has a soapy feel that makes it very pleasant to work with. A simple 2% addition of bentonite transformed it into a delight to throw! That only increased the drying shrinkage by about 0.5%. A potters wheel can thus be seen as a laboratory instrument, giving one the ability to make a comparative assessment like this (that no pairs of datasheets full of numbers will reveal). …
-
Tap picture for full size and resolution
 M-4 Fireclay fired bars
M-4 Fireclay fired bars
Cone 10R top, cone 11 oxidation downward below that. …
-
Tap picture for full size and resolution
 PBX Fireclay fired test bars
PBX Fireclay fired test bars
Cone 10 reduction (top), cone 10 down to 6 oxidation below that (top to bottom). A refractory material. …
-
Tap picture for full size and resolution
 Pine Lake fireclay lab test bars
Pine Lake fireclay lab test bars
Fired to cone 10R (top) and 7,8,9,10 oxidation (from bottom to top). A refractory material. …
-
Tap picture for full size and resolution
 Plainsman Red Fireclay (Fire-Red)
Plainsman Red Fireclay (Fire-Red)
Top to bottom: Cone 10 reduction, cone 10, 9, 8, 7 oxidation. Fire-Red is a mix of St. Rose Red fireclay, M2 Montana medium fire red clay with some dark burning A1 ball clay. This blend is less refractory than pure St. Rose Red and much more plastic, thus more suitable as a body addition. The color shift between cone 9 and 10 oxidation occurs because of the fluxing action of M2. …
-
Tap picture for full size and resolution
 Reduction and oxidation color difference in a cone 10 red fireclay
Reduction and oxidation color difference in a cone 10 red fireclay
Plainsman FireRed fireclay fired to cone 10R. This shows the effect of reduction where the body is exposed to the kiln atmosphere (very dark burning) and where it is not (inner foot ring). …
-
Tap picture for full size and resolution
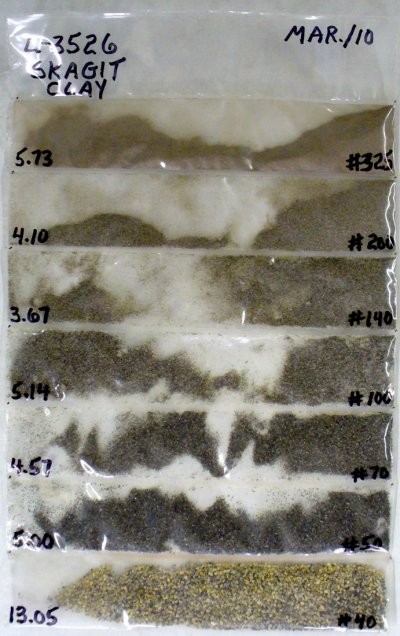 Skagit Fireclay PSD test
Skagit Fireclay PSD test
Particles from each category in a particle size distribution test of Skagit Fireclay …
-
Tap picture for full size and resolution
 Skatgit Fireclay test bars
Skatgit Fireclay test bars
Fired from cone 8-11 and 10 reduction (bottom to top). A refractory material. …
-
Tap picture for full size and resolution
 The stockpile of St. Rose Red fireclay at the Plainsman plant
The stockpile of St. Rose Red fireclay at the Plainsman plant
This is the lump form of Plainsman St. Rose Red clay, as received from the quarry. The bright red color is natural iron oxide. That iron cannot be washed out, it is part of the clay crystal structure. The pigmentation is heavy enough that even when this material is a minor part of a body recipe, that body will still fire to a dark color. The color and high cost of St. Rose Red means that it is always heavily diluted with other clays, many of which are highly plastic. Because St. Rose is non-plastic it is a perfect complement to these. It is also refractory, it melts at a much higher temperature than typical clays. It is mined near St. Rose, Manitoba. …
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
