| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Calculated Thermal Expansion
The thermal expansion of a glaze can be predicted (relatively) and adjusted using simple glaze chemistry. Body expansion cannot be calculated.
Key phrases linking here: calculated thermal expansion, calculated coe - Learn more
Details
Digitalfire Insight-live calculates the coefficient of thermal expansion (CTE) of a glaze by enumerating the percentage analysis, multiplying each value by the expansion factor for that oxide divided by the analysis total times 100 (it also does a secondary calculation by summing the products of the mole% formula and expansion factors). The default oxide thermal expansion values are found on your Preferences page (e.g. SiO2=0.035, K2O=0.331), you can change them. For convenient comparison, these have had the decimal moved 6 places to the right (the actual values for the above are SiO2=0.000000035, K2O=0.000000331). During calculation, Insight-live moves the decimal one more place to the right so the calculated value almost always is a single digit number (with one decimal, e.g. 6.3). Calculated thermal expansions are only intended for comparison, units are not important in this instance (a common unit is in/in/°C).
Results are determined by the set of expansion values (different values are available from different sources) and the method of additive calculation you use (based on analysis or mole%). The value of these sets of numbers is somewhat dubious in that it supposes that a thermal expansion measurement of each oxide in isolation somehow relates to the contribution that each makes as they interact to form a glass. And the numbers from different sources vary widely. And the sources always include only a subset of the oxides, so what do you do for the missing ones? It is kind of "smoke and mirrors". At Digitalfire we did a study of hundreds of frits, comparing the measured thermal expansions with the calculated ones for various sets of numbers. In this setting, they are interacting and the contributions each makes to the total calculated expansion are more real. We found the "West & Garrow" numbers most consistent, not necessarily with the absolute measured values but in comparative magnitudes they calculate to. Please read on for more information on how we rationalize this whole calculation process.
CTE values predicted by calculation are more relative than absolute. They best apply within ‘a system’. In this context, that term is somewhat imprecise. One can stay most ‘within a system’ by tweaking oxide levels in a balanced recipe by adjusting material percentages. Ones goes most ‘out-of-system’ when introducing new oxides from new materials that have different melting patterns. Calculations of CTE are the least helpful when trying a new glaze on which no fired testing has been done.
In special purpose or unbalanced recipes the direction or magnitude of change of the calculated thermal expansion may not be as expected. Also, some oxides, like Li2O or B2O3 do not impose their expansions in a linear fashion. These factors speak well to building experience in the relationships between calculation and fired results within the system you work in.
Another factor relating to the degree to which calculated thermal expansion matches a lab-measured one is the homogeneity of the material. Frits, for example, compared to raw materials, have glass particles of the same chemistry, thus every particle is going to do something predicable during melting. Raw materials, on the other hand, have particles of possibly a dozen different minerals, each having it's own complex melting behavior that is a product of it's mineralogy, chemistry, particle size and shape. The thermal expansion of each changes according to the degree to which its mineral form changes during firing. In addition, these particles interact in complex ways and that further affects their individual expansion characteristics.
As noted, thermal expansion calculations assume a glass, where all oxides have freedom of movement and can impose their proportionate expansion on the whole. Thus, if all the material particles in the glaze are not completely dissolved in the melt the expansion calculation is less accurate (this is one reason why 45 micron silica is so much better than 90 micron). Likewise, clay bodies do not melt like glazes, they undergo complex crystallization and complex phase changes while cooling in the kiln. A glass and a crystal of the same chemistry usually have wildly different physical properties. Consider SiO2: Its percentage may be equal in two bodies, but one may have most of the SiO2 in quartz grains and the other might have it as a molecular component of feldspar and kaolin. These will thus have vastly different thermal expansions.
Another factor is non-melting particles suspended in the glass melt. An example is zircon: its particles impose their expansions differently than if they melt and participate in the glass chemistry.
Can you calculate clay body expansion? No. Thermal expansion calculations assume a glass where all oxides can impose their proportionate expansion on the whole. This does not work for crystalline solids. Clay bodies do not melt like glazes, the oxides do not form a homogeneous glass, they undergo complex crystallization while cooling in the kiln. A glass and crystal of the same chemistry usually have wildly different physical properties. Variations in particle size distribution, particle mineralogy and shape, firing speed, atmosphere and duration of firing all affect the progressive stages of decomposition and play out of interactions that break and build molecular bonds; these variations are evident in the fired product and all beyond the scope of the chemistry. Consider SiO2 oxide content: It may be equal in two bodies, but one may have most of the SiO2 in quartz grains and the other might be in the molecular makeup of feldspar and kaolin. These will, of course, have vastly different thermal expansions. Restated we could say: Clay body expansion is a product of the proportional addition of the expansions of all the mineral particles present adjusted by the degree to which their forms change from base crystalline during firing, the amount of molten glass that forms, the degree to which it dissolves particles, the nature of particle/glass bonding that develops, the degree to which mullite forms or decays and the degree to which the solidifying interparticle glass can impose its expansion on the matrix.
Do you have expansion data for a certain clay body? Are you trying to match that to calculated expansions for glazes? In view of the above it is not going to work, if it does it is purely an accident. Measured thermal expansions are a non-linear line on a graph across 1000 degrees crow-bared into one number. Dilatometer measurements are applicable when they are done by the same team of people on both bodies and glazes and rationalized based on a history of learning to interpret them and observing relationships with real-world fired results of those bodies and glazes. Are these lab-measured numbers useless then? No. They enable you to line up a group of clay bodies from lowest to highest thermal expansion. If you know how your glaze fits on one of the bodies then you are in a position to predict what it will do on another. For example, if your glaze is crazing on body A and body B has a higher thermal expansion, then your glaze should fit that body better.
How does one make practical use of calculated thermal expansion numbers? Remember, they are relative, not absolute. So you use them in that way. If a glaze is crazing that means its thermal expansion is too high. Adjust the formula to bring the calculated expansion down and fire a test and subject it to thermal stress (using the 300F-into-icewater test, for example). If it still crazes move it downward further. If it does not craze then stress test it from ice water to boiling water to check for shivering. With experience, you will learn the amount of change needed and will need to do fewer calculate-test cycles. As a general guide, suppose a glaze crazes badly out of the kiln and the expansion is 7.0. Try to move it down to 6.5. If it crazes only after thermal stress testing then drop it less. Of course, adjusting a glaze to control its thermal expansion will have side effects (e.g. change in the degree of gloss or melt fluidity). Often resourcefulness and plenty of testing are needed to succeed.
Related Information
Adding silica will fix crazing, right? Not here.

This picture has its own page with more detail, click here to see it.
G2926B (center and right) is a clear cone 6 glaze created by simply adding 10% silica to Perkins Studio clear (a glaze that had a slight tendency delay-craze on common porcelains we use). Amazingly that glaze tolerated the silica addition very well, continuing to fire to an ultra gloss crystal clear. That change eliminated the crazing issues on most of our bodies. The cup on the right is one of them, that body is vitreous, near-zero-porosity, and fits most glazes. Why? Because it has 24% silica in the recipe. The center porcelain is also dense and vitreous, but it only has 17% silica, that is why it is crazing this glaze. Then I added 5% more silica to the glaze, it continued to produce an ultra smooth glossy, and applied it to the 17% body on the left. Why did not fix the crazing? That silica addition to the glaze only reduces the calculated expansion from 6.0 to 5.9, clearly not enough to fix the problem. So, the obvious solution seems to be use the porcelain on the right. Are you wondering why adding silica to a body raises its thermal expansion, and adding it to a glaze lowers it? Mineralogy is the reason.
Comparing glaze melt fluidity balls with their chemistries

This picture has its own page with more detail, click here to see it.
Ten-gram GBMF test balls of these three glazes were fired to cone 6 on porcelain tiles. Notice the difference in the degree of melt? Why? You could just say glaze 2 has more frit and feldspar. There is a better explanation, compare these yellow and blue numbers: Glaze 2 and 3 have much more B2O3 (boron, the key flux for cone 6 glazes) and lower SiO2 (silica, it is refractory). But notice that glaze 2 and 3 have the same chemistry, but 3 is melting more? Why? Because of the mineralogy of Gerstley Borate. It release its boron earlier in the firing, getting the melting started sooner. Notice it also stains the glaze amber, it is not as clean as the frit. Notice the calculated thermal expansions: The greater melting of #2 and #3 comes at a cost, their thermal expansions are considerably higher, so they will be more likely to craze. Which of these is the best for functional ware? #1, G2926B (left). Its high SiO2 and enough-but-not-too-much B2O3 make it more durable. And it runs less during firing. And does not craze.
A down side of high feldspar glazes: Crazing!

This picture has its own page with more detail, click here to see it.
This reduction celadon is crazing. Why? High feldspar. Feldspar supplies the oxides K2O and Na2O, they contribute the brilliant gloss and great color but the price is very high thermal expansion. Scores of recipes being traded online are high-feldspar, some more than 50%! There are ways to tolerate the high expansion of KNaO, but the vast majority are crazing on all but high quartz bodies. Crazing is a plague for potters. Ware strength suffers dramatically, pieces leak, the glaze can harbor bacteria and customers return pieces. The simplest fix is to transplant the color and opacity mechanism into a better transparent, one that fits your ware (in this glaze, for example, the mechanism is simply an iron addition). Fixing the recipe may also be practical. A 2:1 mix of silica:kaolin has the same Si:Al ratio as most glossy glazes, this glaze could possibly tolerate 10% of that. That would reduce running, improve fit and increase durability. Failing that, the next step is to substitute some of the high-expansion KNaO, the flux, for the low-expansion MgO, that requires doing some glaze chemistry.
These common Ferro frits have distinct uses in traditional ceramics

This picture has its own page with more detail, click here to see it.
I used Veegum to form 10 gram GBMF test balls and fired them at cone 08 (1700F). Frits melt really well, they do have an LOI like raw materials. These contain boron (B2O3), it is a low expansion super-melter that raw materials don’t have. Frit 3124 (glossy) and 3195 (silky matte) are balanced-chemistry bases (just add 10-15% kaolin for a cone 04 glaze, or more silica+kaolin to go higher). Consider Frit 3110 a man-made low-Al2O3 super feldspar. Its high-sodium makes it high thermal expansion. It works really well in bodies and is great to make glazes that craze. The high-MgO Frit 3249 (made for the abrasives industry) has a very-low expansion, it is great for fixing crazing glazes. Frit 3134 is similar to 3124 but without Al2O3. Use it where the glaze does not need more Al2O3 (e.g. already has enough clay). It is no accident that these are used by potters in North America, they complement each other well (equivalents are made around the world by others). The Gerstley Borate is a natural source of boron (with issues frits do not have).
Why is this crystalline glaze not crazed? Even in the pool at the bottom?

This picture has its own page with more detail, click here to see it.
Because this is Plainsman Crystal Ice, it contains 40% silica (quartz). It also does not vitrify, so as much of the quartz remains undissolved as possible. This produces a body with a much higher thermal expansion so it can put more of a squeeze on the high-expansion glazes used in the crystal glazing process (it is very common for such glazes to be crazed, it is accepted as part of the process).
Insight-Live comparing a glossy and matte cone 6 base glaze recipe
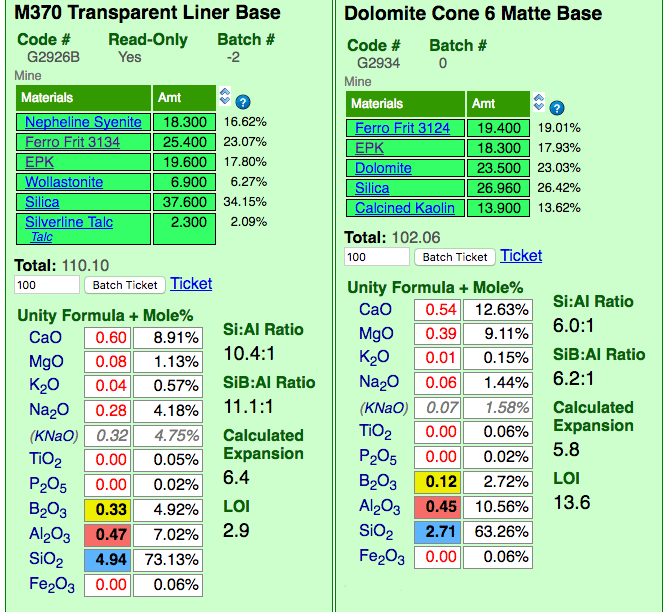
This picture has its own page with more detail, click here to see it.
Insight-live is calculating the unity formula and mole% formula for the two glazes. Notice how different the formula and mole% are for each (the former compares relative numbers of molecules, the latter their weights). The predominant oxides are very different. The calculation is accurate because all materials in the recipe are linked (clickable to view to the right). Notice the Si:Al Ratio: The matte is much lower. Notice the calculated thermal expansion: The matte is much lower because of its high levels of MgO (low expansion) and low levels of KNaO (high expansion). Notice the LOI: The matte is much higher because it contains significant dolomite.
High thermal expansion talc body cannot be COE-calculated

This picture has its own page with more detail, click here to see it.
Talc is employed in low-fire bodies to raise their thermal expansion (to put the squeeze on glazes to prevent crazing). These dilatometer curves make it very clear just how effective that strategy is! The talc body was fired at cone 04 and the stoneware at cone 6. The former is porous and completely non-vitreous and the latter is semi-vitreous. This demonstrates something else interesting: The impracticality of calculating the thermal expansion of clay bodies based on their oxide chemistry. Talc sources MgO and low fire bodies containing it would calculate to a low thermal expansion. But the opposite happens. Why? Because these bodies are composed of mineral particles loosely sintered together. A few melt somewhat, some change their mineral form, many remain unchanged. The body's COE is the additive sum of the proportionate populations of all the particles. Good luck calculating that!
Match calculated COE to dilatometer-measured body COE? No!

This picture has its own page with more detail, click here to see it.
Why? Firing temperature, schedule and atmosphere affect the result. Dilatometers are only useful when manufacturers monitor bodies AND glazes over time and in the same firing conditions. Calculated values for glazes are only relative (not absolute). The best way to fit glazes to your clay bodies is by testing, evaluation, adjustment and retesting. For example, if a glaze crazes, adjust its recipe to bring the expansion down (your account at Insight-live has the tools and guides to do this). Then fire a glazed piece and thermal stress it (300F-to-ice-water IWCT test). If it still crazes, move it further. If you have a base glossy glaze that fits (and made of the same materials), try comparing its calculated expansion as a guide. Can you calculate body expansion from oxide chemistry? Definitely not, because bodies do not melt.
A high expansion glaze is bowing the foot of the bottom bowl

This picture has its own page with more detail, click here to see it.
The glaze has a calculated thermal expansion of 8.8 (because of high KNaO and low SiO2). Very high. It is basically stretched on. These plates are not glazed on the bottom. The glaze on the inside of the upper plate fits, the base is flat. But the glaze on the inside of the lower plate is pulling the base upward. The built-in stresses will eventually cause the piece to fail (likely fracturing into many pieces) if bumped. It is also almost certainly crazing. And the low SiO2 implicates it for leaching. The solution? Reduce the KNaO in favour of MgO and increase the SiO2 as much as possible without compromising the fired character.
Two matte mechanisms: One crazes, the other does not

This picture has its own page with more detail, click here to see it.
These two glazes look the same, they are both cone 6 satin mattes. On the same porcelain. But the matteness "mechanism" of the one on the left, VC71, is a low Si:Al ratio melted by zinc and sodium. The mechanism of the one on the right, G2934, is high MgO melted by enough boron to also have plenty of SiO2 and Al2O3. The "baggage" of the mechanism on the left is high thermal expansion and crazing (drastically reducing strength and providing a space for a germ zoo). If your ware develops this your customers will bring it back for replacement. No change in firing will fix this, the body and glaze are not expansion compatible. Period.
Inbound Photo Links
Links
| Glossary |
Co-efficient of Thermal Expansion
The co-efficient of thermal expansion of ceramic bodies and glazes determines how well they fit each other and their ability to survive sudden heating and cooling without cracking. |
| Glossary |
Oxide System
|
| Glossary |
Glaze Compression
In ceramics, glazes are under compression when they have a lower thermal expansion than the body. A little compression strengthens ware, too much can weaken and even fracture it. |
| Glossary |
Formula Ratios
The ratios of individual or group oxide molecule numbers are indicators of things like fired gloss, durability, melting temperature, balance, tendency to craze, etc. |
| Glossary |
Glaze fit
In ceramics, glaze fit refers to the thermal expansion compatibility between glaze and clay body. When the fit is not good the glaze forms a crack pattern or flakes off on contours. |
| Troubles |
Glaze Shivering
Ask the right questions to analyse the real cause of glaze shivering. Do not just treat the symptoms, the real cause is thermal expansion mismatch with the body. |
| Troubles |
Glaze Crazing
Ask the right questions to analyse the real cause of glaze crazing. Do not just treat the symptoms, the real cause is thermal expansion mismatch with the body. |
| Oxides | MgO - Magnesium Oxide, Magnesia |
| Articles |
Understanding Thermal Expansion in Ceramic Glazes
Understanding thermal expansion is the key to dealing with crazing or shivering. There is a rich mans and poor mans way to fit glazes, the latter might be better. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy