| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Surface Tension
In ceramics, surface tension is discussed in two contexts: The glaze melt and the glaze suspension. In both, the quality of the glaze surface is impacted.
Key phrases linking here: surface tension - Learn more
Details
In ceramics, the issue of surface tension is most evident in two areas: The glaze melt and slurry rheology.
Surface Tension in Glaze Melts
When a glaze is fired, it melts to form a molten silicate glass. This melt exhibits surface tension, a thermodynamic property that reflects the tendency of the liquid to minimize its surface area. In ceramic glazes, surface tension plays a key role in how the melt wets the clay body, releases gases, and levels during firing and cooling.
Wetting and Spreading: Low surface tension promotes wetting of the clay body and allows the glaze to spread more readily across the surface. High surface tension resists wetting and encourages the melt to contract into thicker regions, contributing to beading, crawling, and poor coverage.
Bubble Release: Low surface tension helps bubbles break through the surface and escape. High surface tension can cause bubbles to remain trapped beneath the surface or re-seal after breaking, leading to pinholing and blisters.
Levelling and Surface Smoothness: Surface tension provides the driving force for surface smoothing. However, the extent and rate of levelling are controlled primarily by viscosity (melt mobility). A glaze with high surface tension but low viscosity can still level well, while a glaze with low surface tension but high viscosity may freeze before smoothing is complete.
Surface tension in silicate melts is strongly influenced by oxide chemistry, as different oxides alter the structure and bonding of the glass network.
Oxides that tend to increase surface tension and/or stiffen the melt (approximate, system- and concentration-dependent, rightward have higher surface tension and are thus commonly associated with matteness, opacity, increased melt stiffness, reduced wetting and bubble mobility):
MgO, Al2O3, ZrO2, ZnO, CaO, SnO2, BaO, SrO
Oxides that tend to reduce surface tension (lowest surface tension rightward; these generally promote wetting, improve levelling, enhance bubble release, and increase melt fluidity):
PbO, B2O, K2O, Na2O, Li2O
SiO2, Fe2O3 typically exhibit moderate effects on surface tension, depending on concentration and the surrounding oxide balance.
ZnO is chemically distinctive in that it can function as a strong flux while also increasing melt viscosity, modifying surface behavior and contributing to matte or semi-matte effects in some systems.
Practical Implications of high surface tension + high viscosity are beading, crawling, poor bubble escape, matte or uneven surfaces. Low surface tension + low viscosity brings good wetting, smooth levelling, and glossy surfaces. High surface tension + low viscosity can still level well, but may show edge pullback or thick/thin pooling. Low surface tension + high viscosity implies good coverage, but may freeze before smoothing is complete (satin/matte surfaces).
Surface tension is strongly implicated in glaze blistering. Blisters happen where the molten glaze resists the breaking of bubbles at the surface, these or their unhealed remnants often survive to the fired piece. Strangely, glazes of a more fluid melt can exhibit this problem more, especially if they contain high-surface-tension oxides. It is the surface tension and melt fluidity that enable bubbles to expand without bursting. The solution can be a slower cooling through the temperature at which the increasing temperature-induced viscosity has the power to break the film. Even better, oxides of lower surface tension can be substituted for those of higher.
Surface Tension of Glaze Slurries
On flat surfaces, glaze laydown is not normally an issue, but slurries are suspensions and can resist adhesion and give poor coverage on irregular surfaces (e.g. acute angles, incised or stamped decoration or labelling). Surfactants are available to reduce the surface tension of aqueous glaze systems, they deagglomerate particles and help their homogeneous dispersion over the surface. Polyethylene glycol and Glycerin are considered wetting agents also.
Related Information
Two transparents having opposite melt fluidity/surface tension balances

This picture has its own page with more detail, click here to see it.
This cone 04 flow tester compares two commercial low-fire transparent glazes. Their different approaches to the chemistry are revealed by these melt flows. While 3825B appears to have a higher melt fluidity, its higher surface tension is the real story. This is demonstrated by how the flow meets the runway at a perpendicular angle. Notice that A, by contrast, meanders down the runway in a broad, flat and relatively bubble-free river. Low-fire glazes must pass many more bubbles than their high-temperature counterparts, the low surface tension of A aids in that. A is Amaco LG-10. B is Crysanthos SG213 (Spectrum 700 behaves similarly, although flowing less). Both have advantages and disadvantages and are worth testing in your application.
Surface tension differences between two glazes

This picture has its own page with more detail, click here to see it.
Both are low fire transparents. In a melt fluidity test they flow similarly. But here, where a 10 gram ball has melted down onto the tile (a GBMF test), differences in surface tension are clearly evident by the angle at which the edge of the glaze meets the tile.
The perfect storm of high surface tension and high LOI: Blisters.

This picture has its own page with more detail, click here to see it.
An example of how calcium carbonate can cause blistering as it decomposes during firing. This is a cone 6 Ferro Frit 3249 based transparent (G2867) with 15% calcium carbonate added (there is no blistering without it). Calcium carbonate has a very high loss on ignition (LOI) and for this glaze, the gases of its decomposition are coming out at the wrong time. While there likely exists a firing schedule that takes this into account and could mature it to a perfect surface, the glaze is high in MgO, it has a high surface tension. That is likely enabling bubbles to form and hold better.
The difference that caused blistering: Firing schedule!

This picture has its own page with more detail, click here to see it.
These are the same glaze, same thickness, Ulexite-based G2931B glaze, fired to cone 03 on a terra cotta body. The one on the right was fired from 1850F to 1950F at 100F/hr, then soaked 15 minutes and shut off. The problem is surface tension. Like soapy water, when this glaze reaches cone 03 the melt is quite fluid. Since there is decomposition happening within the body, gases being generated vent out through surface pores and blow bubbles. I could soak at cone 03 as long as I wanted and the bubbles would just sit there. The one on the left was fired to 100F below cone 03, soaked half an hour (to clear micro-bubble clouds), then at 108F/hr to cone 03 and soaked 30 minutes, then control-cooled at 108F/hr to 1500F. During this cool, at some point well below cone 03, the increasing viscosity of the melt becomes sufficient to overcome the surface tension and break the bubbles. If that point is not traversed too quickly, the glaze has a chance to smooth out (using whatever remaining fluidity the melt has). Ideally I should identify exactly where that is and soak there for a while.
Carbonate gassing can cause glaze blisters

This picture has its own page with more detail, click here to see it.
An example of how a carbonate can cause blistering. Carbonates produce gases during decomposition. This glaze (G2415B) contains 10% lithium carbonate, which likely pushes the initial melting temperature down toward the most active decomposition temperatures.
Blistering in a cone 6 white variegated glaze. Why?
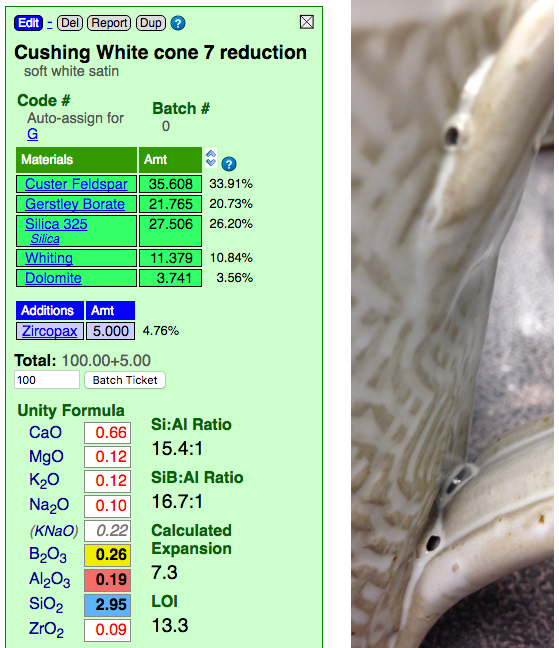
This picture has its own page with more detail, click here to see it.
This glaze creates the opaque-with-clear effect shown (at cone 7R) because it has a highly fluid melt that thins it on contours. It is over fired. On purpose. That comes with consequences. Look at the recipe, it has no clay at all! Clay supplies Al2O3 to glaze melts, it stabilizes it against running off the ware (this glaze is sourcing some Al2O3 from the feldspar, but not enough). That is why 99% of studio glazes contain clay (both to suspend the slurry and stabilize the melt). Clay could likely be added to this to increase the Al2O3 enough so the blisters would be less likely (it would be at the cost of some aesthetics, but likely a compromise is possible). There is another solution: A drop-and-soak firing. See the link below to learn more. One more observation: Look how high the LOI is. Couple that with the high boron, which melts it early, and you have a fluid glaze melt resembling an Aero chocolate bar!
Inbound Photo Links
 High and low surface tension Frits |
 Why do gummed dipping glazes do this as they dry? How to fix it. |
Links
| Troubles |
Crawling
Ask yourself the right questions to figure out the real cause of a glaze crawling issue. Deal with the problem, not the symptoms. |
| Troubles |
Glaze Blisters
Questions and suggestions to help you reason out the real cause of ceramic glaze blistering and bubbling problems and work out a solution |
| Glossary |
Glaze Crawling
A ceramic glaze fault that occurs during firing of the ware, the molten glaze pulls itself into islands leaving bare patches of body between. |
| Glossary |
Glaze Blisters
Blistering is a common surface defect that occurs with ceramic glazes. The problem emerges from the kiln and can occur erratically in production. And be difficult to solve. |
| Glossary |
Glaze Bubbles
Suspended micro-bubbles in ceramic glazes affect their transparency and depth. Sometimes they add to to aesthetics. Often not. What causes them and what to do to remove them. |
| Glossary |
Melt Fluidity
Ceramic glazes melt and flow according to their chemistry, particle size and mineralogy. Observing and measuring the nature and amount of flow is important in understanding them. |
| Glossary |
Rutile Blue Glazes
A type of ceramic glaze in which the surface variegates and crystallizes on cooling in the presence of titanium and iron (usually sourced by rutile) |
| Glossary |
Fluid Melt Glazes
Fluid melt glazes and over-melting, over fired, to the point that they run down off ware. This feature enables the development of super-floss and cyrstallization. |
| Glossary |
Ceramic Glaze Defects
Ceramic glaze defects include things like pinholes, blisters, crazing, shivering, leaching, crawling, cutlery marking, clouding and color problems. |
| Articles |
A Low Cost Tester of Glaze Melt Fluidity
Use this novel device to compare the melt fluidity of glazes and materials. Simple physical observations of the results provide a better understanding of the fired properties of your glaze (and problems you did not see before). |
| Recipes |
G3806C - Cone 6 Clear Fluid-Melt transparent glaze
A base fluid-melt glaze recipe developed by Tony Hansen. With colorant additions it forms reactive melts that variegate and run. It is more resistant to crazing than others. |
| URLs |
https://en.wikipedia.org/wiki/Surfactant
Surfactants at Wikipedia |
| URLs |
https://www.zschimmer-schwarz.com/en/ceramic-auxiliaries/tiles/glaze-additives/wetting-agents
Wetting agents from zschimmer-schwarz.com: Surfactants that reduce the surface tension of aqueous glaze systems. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
