| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Formula Ratios
The ratios of individual or group oxide molecule numbers are indicators of things like fired gloss, durability, melting temperature, balance, tendency to craze, etc.
Key phrases linking here: formula ratios - Learn more
Details
Conceptually we consider fired glazes as having a structure of oxides held together by molecular bonds. Ten major oxides likely make up 98% of all base glazes. Each oxide contributes specific characteristics to the glass and they interact in predictable ways. By rationalizing their absolute values and balance with how glazes fire we can tune individual properties (e.g. melting temperature, thermal expansion, degree of matteness or gloss).
Digitalfire Insight-live shows several ratios as part of its chemistry calculation of a batch recipe.
Si:Al Ratio: The number of SiO2 molecules compared to the number of Al2O3 (in the fired formula). It is an indicator of glaze matteness (where the matteness mechanism is a low Si:Al ratio and the glaze is melted well enough that sources of SiO2 have all melted or dissolved in).
SiB:Al Ratio: The number of SiO2 and B2O3 molecules compared to the number of Al2O3 (in the fired formula). The SiB:Al ratio is thus higher than the Si:Al ratio in a glaze. Since middle-fire glazes usually have less than 5% molar of B2O3, the difference is not great. Since B2O3 also acts as a glass former (in addition to SiO2) it is logical to group the two and compare that to the Al2O3 content in low and medium fire glazes (since these almost always contain B2O3). Glazes that contain significant boron can dissolve more Al2O3 into solution and still stay glossy, so a matte glaze containing boron would not have a higher SiB:Al ratio. While it is not typical to formulate high alumina mattes at low fire, other matteness mechanisms perform best having higher SiB:Al ratios.
R2O:RO Ratio: Alkalis:Alkaline earths or (Li2O+Na2O+K2O : MgO+CaO+SrO+BaO).
Consider an example: There is 5.0 SiO2 and 0.5 Al2O3 in a formula. The ratio is thus 5.0:0.5 or 10:1, or just 10. This ratio is significant in stoneware glazes because high silica tends to produce glossy glazes (when alumina is low) and high alumina creates matte glazes (when silica is low). It thus follows that the lower the Si:Al ratio is - the more matte a glaze will be (there are other matteness mechanisms like high CaO or MgO, under-firing a boron frit or a fluid melt that grows micro crystals). Since Al2O3 adds toughness and durability to glazes it is often advisable to have more alumina in the formula. Glazes can still be glossy even at an 8:1 or lower (especially if boron is present).
Related Information
Insight-Live comparing a glossy and matte cone 6 base glaze recipe
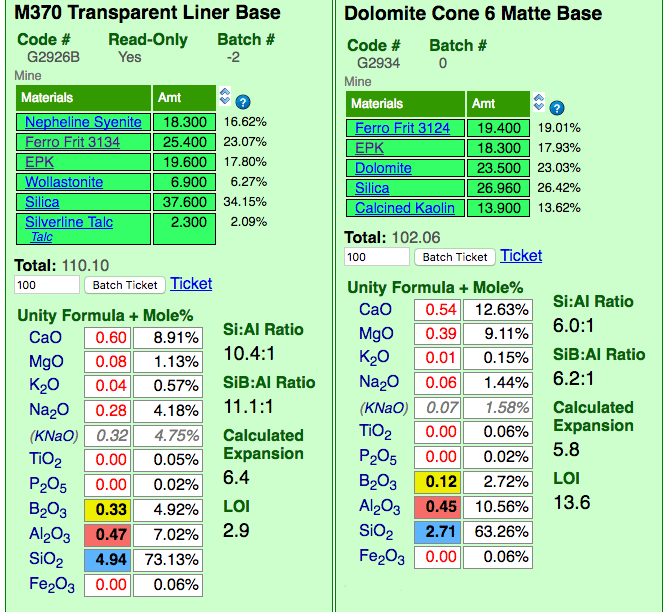
This picture has its own page with more detail, click here to see it.
Insight-live is calculating the unity formula and mole% formula for the two glazes. Notice how different the formula and mole% are for each (the former compares relative numbers of molecules, the latter their weights). The predominant oxides are very different. The calculation is accurate because all materials in the recipe are linked (clickable to view to the right). Notice the Si:Al Ratio: The matte is much lower. Notice the calculated thermal expansion: The matte is much lower because of its high levels of MgO (low expansion) and low levels of KNaO (high expansion). Notice the LOI: The matte is much higher because it contains significant dolomite.
One way for an ultra clear at low fire: Magnesia-alkali, low Si:Al ratio, more boron.

This picture has its own page with more detail, click here to see it.
On the left is G2931J, a zinc alkali fluxed and high Si:Al ratio glaze. Those look like micro-bubbles but they are much more likely to be micro-crystals (high-zinc and high-silica is the mechanism for crystalline glazes). G2931K on the right has much more boron, double the Al2O3, less SiO2 and is magnesia-alkali instead of zinc-alkali. It is the product of dozens of tests to find an ultra-clear having a glassy smooth surface. This particular chemistry, although having only a 6:1 SiO2:Al2O3 ratio is ultra-gloss. In addition, is has low expansion, will fast fire and the boron is not high enough to compromise the hardness.
Links
| Glossary |
Calculated Thermal Expansion
The thermal expansion of a glaze can be predicted (relatively) and adjusted using simple glaze chemistry. Body expansion cannot be calculated. |
| Glossary |
Silica:Alumina Ratio
A formula ratio used to evaluate and predict firing properties in ceramic glazes. |
| Glossary |
LOI
Loss on Ignition is a number that appears on the data sheets of ceramic materials. It refers to the amount of weight the material loses as it decomposes to release water vapor and various gases during firing. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
