| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Reduction Firing
A method of firing stoneware where the kiln air intakes and burners are set to restrict or eliminate oxygen in the kiln such that metallic oxides convert to their reduced metallic state.
Key phrases linking here: cone 10 reduction, reduction firing, reduction fired, reduction-fired, cone 10r - Learn more
Details

A kiln atmosphere that is deficient in free oxygen. In traditional ceramics, reduction firing requires a specially designed fuel-fired kiln that restricts the flow of incoming air so there is enough to burn the fuel and no more (in some cases it is restricted so that is actually less than enough to introduce carbon into the atmosphere). This condition is accomplished in gas kilns by increasing back-pressure or reducing the amount of primary air available to each burner. The result is an increase in gases like carbon, hydrogen and CO. These are very aggressive in wanting to combine with oxygen, they steal it from within bodies and glazes. Hydrogen is small and particularly oxygen-hungry.
Reduction firing produces different colors and visual effects because metallic oxides willing to give up oxygen convert to their reduced or more metallic form. The associated effects include some impossible or difficult to achieve in oxidation. Good examples are copper which burns red (it fires green in oxidation), iron which becomes a powerful flux and produces a wide range of intense and earth-tone browns (it is refractory in oxidation), celadon greens and blues and dolomite mattes. A particularly interesting effect is iron speckling in clay bodies. In fact, because almost all natural clays contain iron, reduction firing normally gives completely different clay surface effects than oxidation.

Gas kilns are typically large and heavy compared to the weight-of-product being fired in them. That means that firing schedules can be more consistent from firing to firing. Often much more consistent. At Plainsman Clays we use the C10RPL schedule. Thus, once a particular glaze effect is working well (e.g. a silky surfaced dolomite matte), then as long as the glaze recipe and materials and glazing process do not change, it will fire the same time after time. The rate-of-rise and rate-of-cool in small electric kilns, by contrast, varies with the load of ware being fired. It is actually possible to produce a reduction-quality dolomite matte at cone 6 oxidation, but only if the cooling rate of the firing is tuned to produce it. And, close attention is needed to keep that rate consistent from firing-to-firing. It requires programming the kiln to control the rate of fall and not firing loads having a natural fall slower than that programmed rate.
Many people fire their gas kilns up in oxidation but at two places in the ramp (e.g. cone 06, 10) they reduce the kiln for a period (for body and glaze reductions). Others begin reduction firing around cone 06 and continue it to the end. Many people do a period of oxidation at the end of a reduction firing to clean the atmosphere and soak the glaze to heal bubbles that result from the active volatilization (an accompanying bubble formation and surface disruption) that reduction induces. In many cases, color breaks in glazes are a result of localized reoxidation of the melt surface (the effect depends on glaze thickness and evenness of coverage). Tenmoku glazes are an example of this, the brown thinner areas are oxidized.
An oxygen probe provides a direct measurement of the amount of reduction and enables one to more easily maintain the critical balance between oxidation and incomplete combustion. While these devices are quite expensive there are very few people employing this process that are not at least planning to get one (rather than just eyeballing the flame).
Reduction firings are not without hazard. Any form of incomplete combustion can generate smoke and deadly gases (CO, for example, is colorless and odorless). It is important that gas kilns be vented well, and if possible, that a CO alarm be installed.
Related Information
Reduction speckle: a product of iron particles in the body

This picture has its own page with more detail, click here to see it.
In reduction firing, where insufficient oxygen is present to oxidize the iron, natural iron pyrite particles in the clay convert to their metallic form and melt. The nature of the decorative speckled effect depends on the size of the particles, the distribution of sizes, their abundance, the color of the clay and the degree to which they melt. The characteristics of the glaze on the ware (e.g. degree of matteness, color, thickness of application, the way it interacts with the iron) also have a big effect on the appearance.
Plainsman Fire-Red reduction fired vase

This picture has its own page with more detail, click here to see it.
Fire-Red clay is a 50:50 mix of St. Rose Red and M2 with 10% A1 bentonitic clay. The St. Rose is a red fireclay, not useful on its own in reduction firing (because it is too refractory). The M2 supplies iron staining but also natural feldspar to mature the body enough to make it fire strong. The A1 clay contributes iron pyrite speckle and plasticity (in heavy reduction, with a little more feldspar added, this body can fire metallic). The glaze is G2571A bamboo. This piece exhibits the trimming ring (one third of the way up) which divides the thrown surface (upper) with the trimmed one (lower).
Iron speckled cone 10 reduction stoneware with dolomite glaze

This picture has its own page with more detail, click here to see it.
Plainsman H443. By Tony Hansen. The silky matte yet functional surface of this type of glaze, combined with the iron speckle from the body bleeding through it, has been a key reason why many have sought the cone 10R temperature range for pottery.
Additions of iron oxide are coloring, fluxing and crystallizing this base transparent

This picture has its own page with more detail, click here to see it.
Iron oxide is an amazing glaze addition in reduction. Here, I have added it to the G1947U transparent base. It produces green celadons at low percentages. Still transparent where thin, 5% produces an amber glass (and the iron reveals its fluxing power). 7% brings opacity and tiny crystals are developing. By 9% color is black where thick, at 11% where thin or thick - this is “tenmoku territory”. 13% has moved it to an iron crystal (what some would call Tenmoku Gold or Teadust), 17% is almost metallic. Past that, iron crystals are growing atop others. These samples were cooled naturally in a large reduction kiln using the C10RPL firing schedule, the crystallization mechanism would be heavier if it were cooled more slowly (or less if cooled faster). The 7% one in this lineup is quite interesting, a minimal percentage of cobalt-free black stain could likely be added to create an inexpensive and potentially non-leaching jet-black glossy.
Cone 10 Reduction, the home of an amazing oxide: Iron

This picture has its own page with more detail, click here to see it.
It is a powerful glaze flux, variegator and crystalizer, a colorant of many characters in bodies and glazes and a specking agent like no other. And it is safe and cheap!
Copper red reduction glaze at cone 9 reduction
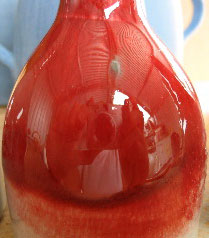
This picture has its own page with more detail, click here to see it.
The color red is difficult to achieve in ceramics. In reduction firing copper can turn many dazzling shades of red.
FeO (iron oxide) is a very powerful flux in reduction

This picture has its own page with more detail, click here to see it.
This cone 10R glaze, a tenmoku with about 12% iron oxide, demonstrates how iron turns to a flux, converting from Fe2O3 to FeO, in reduction firing and produces a glaze melt that is much more fluid. In oxidation, iron is refractory and does not melt well (this glaze would be completely stable on the ware in an oxidation firing at the same temperature, and much lighter in color).
How reduction firing can affect glaze color

This picture has its own page with more detail, click here to see it.
An example of how the same dolomite cobalt blue glaze fires much darker in oxidation than reduction. But the surface character is the same. A different base glaze having the same colorant might fire much more similar. The percentage of colorant can also be a factor in how similar they will appear. The identity of the colorant is important, some are less prone to differences in kiln atmosphere. Color interactions are also a factor. The rule? There is none, it depends on the chemistry of the host glaze, which color and how much there is.
The same glaze in reduction (left) and oxidation at cone 10

This picture has its own page with more detail, click here to see it.
It is not just iron oxide that changes character from oxidation to reduction. Of course, cobalt can fire to a bright blue in oxidation also, but this will only happen if its host glaze is glossy and transparent. In this case the shift to reduction has altered the character of the glaze enough so that its matte character subdues the blue.
Difference between oxidation and reduction! GR10-C matte on Plainsman H443

This picture has its own page with more detail, click here to see it.
Same body, same glaze. Left is cone 10 oxidation, right is cone 10 reduction. What a difference! This is a Ravenscrag-Slip-based recipe on a high-fire iron stoneware. In reduction, the iron oxide in the body and glaze darkens (especially the body) and melts much more. The behavior of the tin oxide opacifier is also much different (having very little opacifying effect in reduction).
Reduction Polar Ice vs. Oxidation Polar Ice

This picture has its own page with more detail, click here to see it.
Polar Ice (Plainsman Clays) has been fired to cone 10R (left). This is beyond the recommended cone 6 range, but it worked well in this instance. The result is even more translucency and a translucency of a different character: blue! This looks much more like real blue polar ice.
The same bamboo glaze on light and dark bodies at cone 10R!

This picture has its own page with more detail, click here to see it.
This is G2571A with iron and rutile on Plainsman H550 and H440.
Example of a modern automatic firing reduction gas kiln for use by studio potters

This picture has its own page with more detail, click here to see it.
Courtesy of Bailey Kilns.
A cone 10 reduction firing on the way into the kiln and on the way out

This picture has its own page with more detail, click here to see it.
These are mostly native Plainsman stoneware bodies. The glazes are mostly pure Alberta Slip and pure Ravenscrag Slip (or Ravenscrag with a small talc addition to produce a silky matte). Ware was made by Tony Hansen in June 2017. We use the C10RPL firing schedule.
Blue brick is made by reduction-firing high-iron clays to near vitrification

This picture has its own page with more detail, click here to see it.
A google search for "Staffordshire blue brick" will turn up lots of images and web pages on the topic. Although not really appearing blue on closeup, at a distance the effect is more clear. The clay must have enough iron to both stain it and act as a flux in the reduction kiln atmosphere. The degree of atmospheric consistency inside the kiln will determine the range of colors produced. A potter can achieve this effect by firing a red earthenware in reduction (e.g. to cone 2R), firing a middle-temperature red burning body to cone 6R or adding feldspar to a high temperature reduction red. Additions of iron oxide will enhance the effect, however, thorough testing is needed to achieve the difficult balance of enough iron to get the color but not so much it will over-vitrify and bloat or melt.
A cone 10 Reduction bowl has cracked after continuous use

This picture has its own page with more detail, click here to see it.
This was made by a potter who carefully designed his own body and glaze recipe and obtained a high quality kiln in which to fire a line this line of ware. These pieces are being used in a restaurant and this one has failed. Why? It could be pushing up against the limits of this type of clay body (even at its best). Compared to what typical restaurant ware is made from it has more porosity (demonstrated by the color change around the crack). It likely has entrapped carbon inside. It has lower strength (a big issue restaurant ware which is handled so much). Larger particles of high expansion minerals create vulnerability to sudden temperature changes. If this is all true then all of the bowls will eventually crack.
That being said, what if they do not all crack? Maybe this crack appeared to relieve stresses within the ware from uneven drying or uneven firing (due to technique or uneven thickness). Some bodies expand upon absorbing water, if that is the case with this then the bare clay surfaces (which permit water entry) could be an issue also.
Reduction glaze gone astray returns to color in an oxidation refire

This picture has its own page with more detail, click here to see it.
Refired to 1950F. The recipe is very flux-heavy (high feldspar) dolomite:spodumene matte, zero silica, 4% tin oxide and less than 1% iron oxide. Sounds like crystallization territory. The plate on the left is the way it normally fired. On the right the way it started firing. The mug on the bottom looked like the plate on the right, but look what happened after refiring at 1950F in oxidation! The tin is likely a catalyst for the crystallization that occurred in the original result. Could be a fragile mechanism. This underscores the need for a period of oxidation at the end of a normal reduction firing.
Inadequate air-flow during clearing at the end of a reduction firing

This picture has its own page with more detail, click here to see it.
The clearing period at the end of the reduction firing was done without opening the damper, the gas pressure was simply turned down enough to stop temperature rise. The flame through the damper and the peep holes completely disappeared and this cleared the chamber of carbon immediately. Only one quarter the normal gas pressure was needed to hold temperature. But there was a problem: Inadequate airflow through the chamber to clear carbon build-up in the clays. The issue was most visible on the insides of pieces like this. And it was made worse by another factor: Body carbon trapping of an ultra-transparent early-melting fritted clear glaze that we have been testing.
The same clay fired in oxidation and reduction

This picture has its own page with more detail, click here to see it.
The lower bars are cone 6 and 8 oxidation. The iron converts to a flux in reduction (top bar) and by cone 10 it is overfired enough that it is expanding.
The same clay fired in oxidation and reduction

This picture has its own page with more detail, click here to see it.
This is a highly plastic fine particled ball clay (Plainsman C1). It is also high in lignite, the raw clay is almost black in the wet state. The top bar is fired to cone 10R, the bottom ones at cone 6, 8, 9, 10 (bottom to top). The burnoff of the carbonaceous material is so vigorous that it fractures the bars in oxidation, but in reduction, the effect is much less, likely having occurred during the last 30 minutes of the firing (where clearing is done in oxidation).
Inbound Photo Links
Links
| Glossary |
Oxidation Firing
In ceramics, this term is most often used to refer to kilns firing with an atmosphere having available oxygen to react with glaze and body surfaces during firing |
| Glossary |
Reduction Speckle
A sought-after visual effect that occurs in reduction fired stoneware. Particles of iron pyrite that occur naturally in the clay melt and blossom up through the glaze |
| Glossary |
Kiln Firing
All types of ceramic are fired in a kiln to cement particles together to produce a hard and water and temperature resistant product. |
| Materials |
Iron Pyrite
|
| Materials |
Iron Oxide Red
Red iron oxide is the most common colorant used in ceramic bodies and glazes. As a powder, it is available in red, yellow, black and other colors. |
| Typecodes |
Oxidation to reduction kiln conversions
Many potters working in the electric oxidation process, dream of being able to graduate to fire high temperature reduction. As it turns out, your old electric kiln can be converted. These pages provide detail from multiple people who have done it. The effort they have expended is evidence that the process is viable. |
| Properties | Glaze Variegation |
| Articles |
Firing: What Happens to Ceramic Ware in a Firing Kiln
Understanding more about changes taking place in the ware at each stage of a firing helps tune the curve and atmosphere to produce better ware |
| Articles |
Building a Lindoe Downdraft Gas Kiln
|
| Hazards |
Fumes from gas kilns
|
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy







