| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Soaking
The process of holding a kiln at the final temperature (or at other temperatures) to enable the heat to penetrate the ware or to effect or complete a glaze or body reaction
Key phrases linking here: - Learn more
Details
The practice of holding the kiln at final firing temperature for a period of time. This is usually done to mature the clay (especially if the firing was fast), distribute internal stresses and give the glaze opportunity to flow and heal imperfections. Soaking in a firing schedule should normally be accompanied by a slower approach to final temperature. The advent of electronic kiln controllers has made it possible for anyone to soak at any temperature. Soaking is especially advantageous for glazes with a stiff melt (i.e. low temperature zirconia whites) and for porcelains that require translucency, density, and glassy surfaces. It is also a necessity for heaver kiln loads (to get heat penetration).
Soaking can enable the production of flawless glaze surfaces using bodies or glazes containing coarser particles that generate gases on decomposition that would otherwise leave defects in the glaze surface (especially blisters). However soaking at the final temperature is likely not the answer. It is better to cool the kiln (by 100F for example) and then soak there. The reason this is effective is that the increased viscosity of the melt reaches a point where it is able to overcome the surface tension holding the bubbles in tact (and pop them). Different glazes have different temperatures at which to soak works best.
Soaking (or slow temperature rise) towards the end of the upward firing curve can also be an effective way to reduce the clouds of micro bubbles that would normally be present. This provides enough fluidity to enable bubbles to move through the glaze but enough viscosity to prevent surface tension that would cause bubbles to congregate and produce large domes (that later break to become blisters).
Soaking is also a part of a wide range of firing techniques to achieve variegation, special coloration and crystal growth.
In hi-tech industries, such as those firing almost pure calcined alumina bodies, soaking is a key factor in developing the properties of the matrix, a two-hour firing may finish with a two-hour soak (the soaking however may be done at a much lower temperature).
Related Information
The difficulty of vitrifying ware in electric kilns

This picture has its own page with more detail, click here to see it.
This 1 gallon heavy crock was fired to cone 6 (at 108F/hr during the final 200 degrees) and held 20 minutes (in a electric kiln). The bare clay base should be the color of the top test bar (which has gone to cone 6). Yet, it is the color of the bottom bar (which has gone to cone 4)! That means the base only made it to cone 4. The vertical walls, which we exposed to the direct radiant head of the elements, are the right color (so they made cone 6). It may seem that this problem could be solved by simply firing with a longer hold at cone 6. But again, electric kilns heat by radiation, that shielded and heatsunk base will never get the same thermal treatment as the sidewalls!
Same cone 6 glazes. Same clay. Why is the one on the right pinholing?

This picture has its own page with more detail, click here to see it.
These are thick pieces, they need time for heat to penetrate. Both were soaked 15 minutes at cone 6 (2195F in our test kiln). But the one on the left was control-cooled to 2095F degrees and soaked 45 more minutes. Pinholes and dimples are gone, the clay is more mature and the glaze is glossier and melted better. Why is this better than just soaking longer at cone 6? As the temperature rises the mineral particles decompose and generate gases (e.g. CO2, SO4). These need to bubble through the glaze. But on the way down this activity is ceasing. Whatever is gassing and creating the pinholes will has stopped by 2095F. Also, these are boron-fluxed glazes, they stay fluid all the way down to 1900F (so you could drop even further before soaking).
These two pieces will not mature to the same degree in a firing

This picture has its own page with more detail, click here to see it.
Soak the firing 30 minutes and the mug would mature throughout. But not the planter. Soak 2 hours for the planter and the mug may over-mature and bloat or warp. This is a troublesome issue with electric kilns. Furthermore, they employ radiant heat. That means that sections of ware on the shady side (and the under sides) will never reach the temperature of those on the element side - no matter how long the firing is held at temperature.
What material makes the tiny bubbles? The big bubbles?

This picture has its own page with more detail, click here to see it.
These are two 10 gram GBMF test balls of Worthington Clear glaze fired at cone 03 on terra cotta tiles (55 Gerstley Borate, 30 kaolin, 20 silica). On the left it contains raw kaolin, on the right calcined kaolin. The clouds of finer bubbles (on the left) are gone from the glaze on the right. That means the kaolin is generating them and the Gerstley Borate the larger bubbles. These are a bane of the terra cotta process. One secret of getting more transparent glazes is to fire to temperature and soak only long enough to even out the temperature, then drop 100F and soak there (I hold it half an hour).
Let me count the reasons this glossy white cone 6 glaze is pinholing

This picture has its own page with more detail, click here to see it.
First, the layer is very thick. Second, the body was only bisque fired to cone 06 and it is a raw brown burning stoneware with lots of coarser particles that generate gases as they are heated. Third, the glaze contains zircopax, it stiffens the melt and makes it less able to heal disruptions in the surface. Fourth, the glaze is high in B2O3, so it starts melting early (around 1450F) and seals the surface so the gases must bubble up through. Fifth, the firing was soaked at the end rather than dropping the temperature a little first (e.g. 100F) and soaking there instead.
Bleeding underglaze. Why?

This picture has its own page with more detail, click here to see it.
This cobalt underglaze is bleeding into the transparent glaze that covers it. This is happening either because the underglaze is too highly fluxed, the over glaze has too high of a melt fluidity or the firing is being soaked too long. Engobes used under the glaze (underglazes) need to be formulated for the specific temperature and colorant they will host, cobalt is known for this problem so it needs to be hosted in a less vitreous engobe medium. When medium-colorant compounds melt too much they bleed, if too little they do not bond to the body well enough. Vigilance is needed to made sure the formulation is right.
Cone 6 rutile floating blue effect lost. Then regained.

This picture has its own page with more detail, click here to see it.
Left: What GA6-C Alberta Slip rutile blue used to look like. Middle: When it started firing wrong, the color was almost completely lost. Right: The rutile effect is back with a vengeance! What was the problem? We were adjusting firing schedules over time to find ways to reduce pinholing in other glazes and bodies. Our focus was slowing the final stages of firing and soaking there. In those efforts the key firing phase that creates the effect was lost: it happens on the way down from cone 6. This glaze needs a drop-and-soak firing (e.g. cooling 270F from cone 6, soaking, then 150F/hr drop to 1400F).
Melting glaze balls at various temperatures to see when all carbon has been expelled
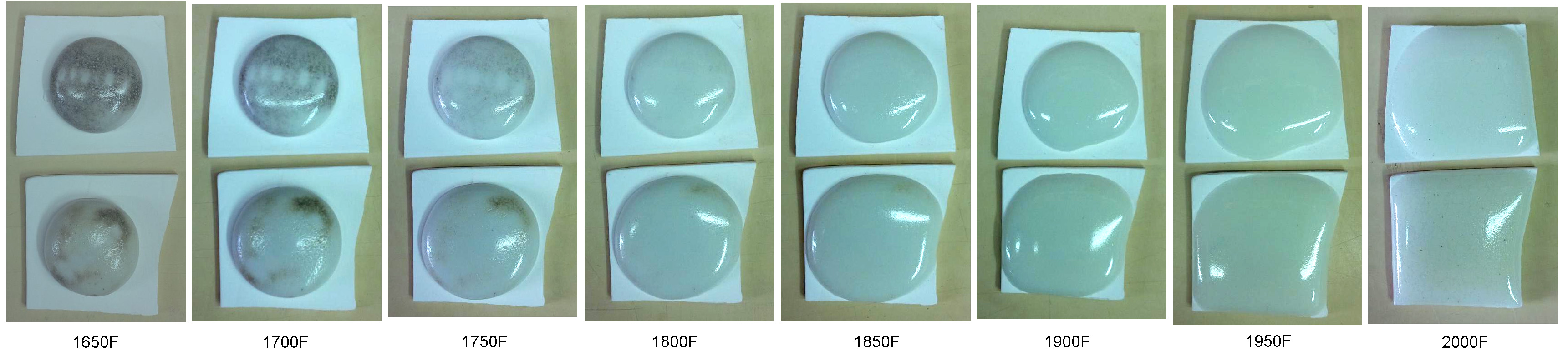
This picture has its own page with more detail, click here to see it.
G1916Q and J low fire ultra-clear glazes (contain Ferro Frit 3195, 3110 and clay) fired across the range of 1650 to 2000F (these were 10 gram GBMF test balls that melted and flattened as they fired). Notice how they soften over a wide range, starting below cone 010 (1700F)! At the early stages carbon material is still visible (even though the glaze has lost 2% of its weight to this point), it is likely the source of the micro-bubbles that completely opacify the matrix even at 1950F (cone 04). This is an 85% fritted glaze, yet it still has carbon - think of what a raw glaze might have! Of course, these specimens test a very thick layer, so the bubbles are expected. But they still can be an issue, even in a thin glaze layer on a piece of ware. So to get the most transparent possible result it is wise to fire tests to find the point where the glaze starts to soften (in this case 1450F), then soak the kiln just below that (on the way up) to fire away as much of the carbon as possible. Of course, the glaze must have a low enough surface tension to release the bubbles, that is a separate issue.
This is when you should program a firing yourself

This picture has its own page with more detail, click here to see it.
This is Polar Ice cone 6 porcelain that has been over fired. The electric kiln was set to do its standard cone 6 fast fire schedule, but a cone in the kiln demonstrates that it fired much higher (perhaps to cone 7 judging by the bend on the cone). This is a translucent frit-fluxed porcelain that demands accurate firing, the over fire has produced tiny bubbles and surface dimples in the glaze. The mug rim has also warped to oval shape. The lesson: If you are firing ware that is sensitive to schedule or temperature, use large cones and adjust if needed. If it fires too hot like this, then program to fire to cone 5 with a longer soak, or cone 5.5 (if possible). Or, program all the steps yourself; that is definitely our preference.
Blisters in a reduction fired rutile glaze

This picture has its own page with more detail, click here to see it.
This is a common problem with these glazes. The visual effect is very compelling but also punishing! Potters experiment with higher bisque firing and soaking during bisque. They try cleaner clay bodies. They employ long hold periods at temperature in the glaze firing. But the problem persists. The solution is actually simpler. These glazes have a high melt fluidity and enough surface tension to hold a bubble static during soaks at temperature (no matter how long you hold it). It is better to cool the kiln somewhat (perhaps 100F) and soak at that temperature. Why? Because the increasing viscosity of the melt overcomes the surface tension that maintains the bubbles. You may need to cool more or less than 100 degrees, but start with that.
Inbound Photo Links
 More carbon needs to burn out than you might think! |
Links
| Articles |
Electric Hobby Kilns: What You Need to Know
Electric hobby kilns are certainly not up to the quality and capability of small industrial electric kilns, being aware of the limitations and keeping them in good repair is very important. |
| Media |
Manually program your kiln or suffer glaze defects!
To do a drop-and-hold firing you must manually program your kiln controller. It is the secret to surfaces without pinholes and blisters. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
