| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Ferro Frit 3195
Alternate Names: F3195
Description: Leadless High Borax/calcia for glaze
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 11.36% | 0.69 | |
| Na2O | 5.69% | 0.31 | |
| B2O3 | 22.62% | 1.10 | |
| Al2O3 | 11.98% | 0.40 | |
| SiO2 | 48.35% | 2.73 | |
| Oxide Weight | 339.82 | ||
| Formula Weight | 339.82 | ||
Notes
This is a USA pottery frit, it is very common in North America and there are equivalents available around the world. Ferro now calls it Frit 3195-2.
Like Frit 3124, this can be a complete cone 06-02 leadless pottery glaze with the addition of a little kaolin to suspend. Adding more kaolin and silica will produce glazes suitable for higher temperatures. And 85:15 frit:kaolin mix of this produces a transparent cone 04 base. This 85:15 blend is not mirror-glossy, having a slightly matte finish. Since Frit 3195 is middle-of-the-road thermal expansion the 85:15 mix will fit most bodies. The G1916Q expansion-adjustable recipe employs this frit (mixed with Frit 3110 and 3249).
This frit works well at stoneware temperatures as a source of boron, all the other oxides it supplies are needed and none are excessive.
If used in too high a percentage, this frit can push the boron too high for use in underglaze colors.
A chart from Ferro 1962 listed Na2O 10.3, B2O3 15.8.
Fusion Frit F-2 has proven a good substitute for this.
Related Information
A frit softens over a wide temperature range

This picture has its own page with more detail, click here to see it.
Raw materials often have a specific melting temperature (or they melt quickly over a narrow temperature range). We can use the GLFL test to demonstrate the development of melt fluidity between a frit and a raw material. On the left we see five flows of boron Ferro Frit 3195, across 200 degrees F. Its melting pattern is slow and continuous: It starts flowing at 1550F (although it began to turn to a glass at 1500F) and is falling off the bottom of the runway by 1750F. The Gerstley Borate (GB), on the other hand, goes from no melting at 1600F to flowing off the bottom by 1625F! But GB has a complex melting pattern, there is more to its story. Notice the flow at 1625F is not transparent, that is because the Ulexite mineral within GB has melted but its Colemanite has not. Later, at 1700F, the Colemanite melts and the glass becomes transparent. Technicians call this melting behaviour "phase transition", that does not happen with the frit.
These common Ferro frits have distinct uses in traditional ceramics

This picture has its own page with more detail, click here to see it.
I used Veegum to form 10 gram GBMF test balls and fired them at cone 08 (1700F). Frits melt really well, they do have an LOI like raw materials. These contain boron (B2O3), it is a low expansion super-melter that raw materials don’t have. Frit 3124 (glossy) and 3195 (silky matte) are balanced-chemistry bases (just add 10-15% kaolin for a cone 04 glaze, or more silica+kaolin to go higher). Consider Frit 3110 a man-made low-Al2O3 super feldspar. Its high-sodium makes it high thermal expansion. It works really well in bodies and is great to make glazes that craze. The high-MgO Frit 3249 (made for the abrasives industry) has a very-low expansion, it is great for fixing crazing glazes. Frit 3134 is similar to 3124 but without Al2O3. Use it where the glaze does not need more Al2O3 (e.g. already has enough clay). It is no accident that these are used by potters in North America, they complement each other well (equivalents are made around the world by others). The Gerstley Borate is a natural source of boron (with issues frits do not have).
Can frits be partially soluble? Yes, and here is what that means.

This picture has its own page with more detail, click here to see it.
These 1 mm-sized crystals were found precipitated in a couple of gallons of glaze containing 85% Ferro Frit 3195. They are cubical, hard and insoluble. Why and how to do they form? Many frits are slightly soluble, the degree to which they are is related to the length of time the glaze is in storage, the temperature, the electrolytes and solubles in the water, interactions with other material particles present and the diligence of the manufacturer in mixing, correctly achieving the target chemistry and firing. The solutes interact or saturate to form insoluble species that crystallize and precipitate out as you see here. These crystals can be a wide range of shapes and sizes and come from leaded and unleaded frits. In industry this issue is not generally a problem because glazes are used soon after being made.
Same glaze, same kiln, same clay: The right one crystallized. Why?

This picture has its own page with more detail, click here to see it.
Well, actually they are not exactly the same. This is 80% Alberta Slip and 20% frit. But the frit on the left is Ferro 3195 and on the right is 3134. By comparing the calculated chemistry for these two we can say that the likely reason for the difference is the Al2O3 content. Frit 3134 has almost none whereas 3195 has 12%. Al2O3 stiffens the glaze melt, that impedes crystal growth. And it stabilizes the melt against running during firing. Frit 3195 is thus much more "like a glaze" than is 3134, it is what Alberta Slip needs to melt as a transparent glass under normally cooling in the kiln.
Stoneware from your terra cotta body? It is very possible.

This picture has its own page with more detail, click here to see it.
Some terra cotta clays can be used to produce stoneware by firing them a few cones higher. Terra Cottas are almost always nowhere near vitrified at their traditional cone 04-06 temperatures, so they can often stand much higher firing. However, clear glazes do not usually work well in higher firing since products of decomposition from the vitrifying body fill them will micro bubbles, clouding the surface. In addition, the body turns dark brown under clear glazes. But with a white glaze, these are not a problem. This is Plainsman L210 fired to cone 2. The glaze is 80% Frit 3195, 20% kaolin and 10-12% zircopax, it fires to a brilliant flawless surface.
A Redart cone 03 body shines when it come to ease of glaze fit

This picture has its own page with more detail, click here to see it.
These bowls are fired at cone 03. They are made from 80 Redart, 20 Ball clay. The glazes are (left to right) G1916J (Frit 3195 85, EPK 15), G191Q (Frit 3195 65, Frit 3110 20, EPK 15) and G1916T (Frit 3195 65, Frit 3249 20, EPK 15). The latter is the most transparent and brilliant, even though that frit has high MgO. The center one has a higher expansion (because of the Frit 3110) and the right one a lower expansion (because of the Frit 3249). Yet all of them survived a 300F to icewater IWCT test without crazing. This is a testament to the utility of Redart at low temperatures. A white body done at the same time crazed the left two.
Melt fluidity and coverage: RedArt Slip vs. Albany Slip vs. Alberta Slip

This picture has its own page with more detail, click here to see it.
These three melt flows and mugs were fired at cone 6 (using the C6DHSC firing schedule). The benchmark recipe is 80% clay and 20% Ferro Frit 3195 (our standard GA6-B recipe).
-The center melt flow (and matching buff stoneware mug below) employ the original Albany Slip.
-The one on the right employs Alberta Slip. Notice that, although having a very similar melt flow, it needs an iron oxide addition to darken the color (e.g. 2%).
-The one on the far left uses an Albany Slip substitute made from 80% Redart, 6.5% calcium carbonate, 6.5% dolomite and 6.5% nepheline syenite (our code L3613D). The chemistry of RedArt is different enough from Albany that some compromises were needed to avoid over-supplying the iron even more (and firing darker yet). Although this Redart version runs in a very similar pattern on the melt flow, the character of the glaze on the mug reveals it needs a little more melting (increasing the frit percentage would take care of that).
Was that batch of frit 3195 really bad?

This picture has its own page with more detail, click here to see it.
After a customer experienced blistering with a transparent glaze on a terra cotta body, suspicion was raised that the batch of frit was bad. Ferro even admitted there was an issue. But we decided to test, sampling about 20 of the 50 lb bags and combining that to make these tests. Upper left is a GLFL test, if compares the melt flow of an old batch of frit 3195 with the questioned one, fired at 1750F. Although the questioned batch does run a little more, a more important difference is in the bubble development (look closer), perhaps there is some fluorine contamination. A GBMF test compares the two samples on the bottom left (10 gram balls melt downward onto a sample tile). Again, the questioned batch is melting and bubbling a little more. We made two glazes: Cone 6 GA6-B (top right) contains 20% of the frit (the left one uses an old frit batch). The cone 05 G1916QL1 glaze (bottom right, it contains 60% of the frit) uses the questioned frit and works well on both the white and red bodies. So, although it might not be working for some, we determined that the frit is OK! This being said, this test assumes all the bags in the frit batch are be the same, a melt flow test could be done on every bag to be sure.
Melting range is mainly about boron content

This picture has its own page with more detail, click here to see it.
Fired at 1850. Notice that Frit 3195 is melting earlier. By 1950F, they appear much more similar. Melting earlier can be a disadvantage, it means that gases still escaping as materials in the body and glaze decompose get trapped in the glass matrix. But if the glaze melts later, these have more time to burn away. Glazes that have a lower B2O3 content will melt later, frit 3195 has 23% while Frit 3124 only has 14%).
Frit melt fluidity comparison - 1300F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1300F and held for 15 minutes. Some are still burning off carbon (which seems strange). There are two early leaders: Ferro frit 3110 and Fusion frit F75 are starting to deform (they have almost the same chemistry). Amazingly, these two frits have low boron, they rely on high soda as the flux.
Five frits. One kiln at 1850F. Big chemistry drama.

This picture has its own page with more detail, click here to see it.
Five common North American Ferro Frits fired at 1850F on alumina tiles (each started as a 10-gram GBMF test ball and flattened during the firing). At this temperature, the differences are more evident than at 1950F. The degree of melting corresponds mainly, but not only, to the percentage of B2O3 present. Frit 3134 is the runaway leader because it couples that with almost no Al2O3 to stabilize the melt. Notice also the crack pattern, it is also very high in Na2O and thus has a high COE. However, why does Frit 3110 melt so well even though it contains almost no B2O3? Even higher Na2O, it is a powerful flux. Its COE is even higher than Frit 3134, but it is thick enough to have resisted cracking far (but it will in time).
Melting glaze balls at various temperatures to see when all carbon has been expelled
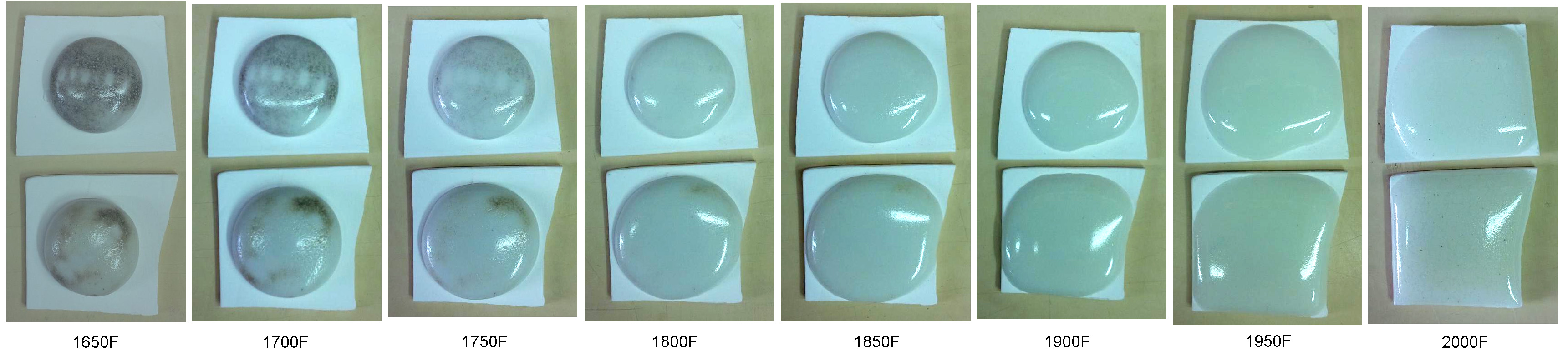
This picture has its own page with more detail, click here to see it.
G1916Q and J low fire ultra-clear glazes (contain Ferro Frit 3195, 3110 and clay) fired across the range of 1650 to 2000F (these were 10 gram GBMF test balls that melted and flattened as they fired). Notice how they soften over a wide range, starting below cone 010 (1700F)! At the early stages carbon material is still visible (even though the glaze has lost 2% of its weight to this point), it is likely the source of the micro-bubbles that completely opacify the matrix even at 1950F (cone 04). This is an 85% fritted glaze, yet it still has carbon - think of what a raw glaze might have! Of course, these specimens test a very thick layer, so the bubbles are expected. But they still can be an issue, even in a thin glaze layer on a piece of ware. So to get the most transparent possible result it is wise to fire tests to find the point where the glaze starts to soften (in this case 1450F), then soak the kiln just below that (on the way up) to fire away as much of the carbon as possible. Of course, the glaze must have a low enough surface tension to release the bubbles, that is a separate issue.
Melt fluidity comparison of frits - 1350F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1350F and held for 15 minutes. Some are still burning off carbon (which seems strange). The two FZ16s are starting to move. Frit 3134 is expanding. 3602 is also starting to melt.
Melt fluidity comparison of frits - 1400F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1400F and held for 15 minutes. Frit 3134 is still expanding. 3602 is also starting to flow. A number of them are shrinking and densifying like a porcelain would.
Melt fluidity comparison of frits - 1450F
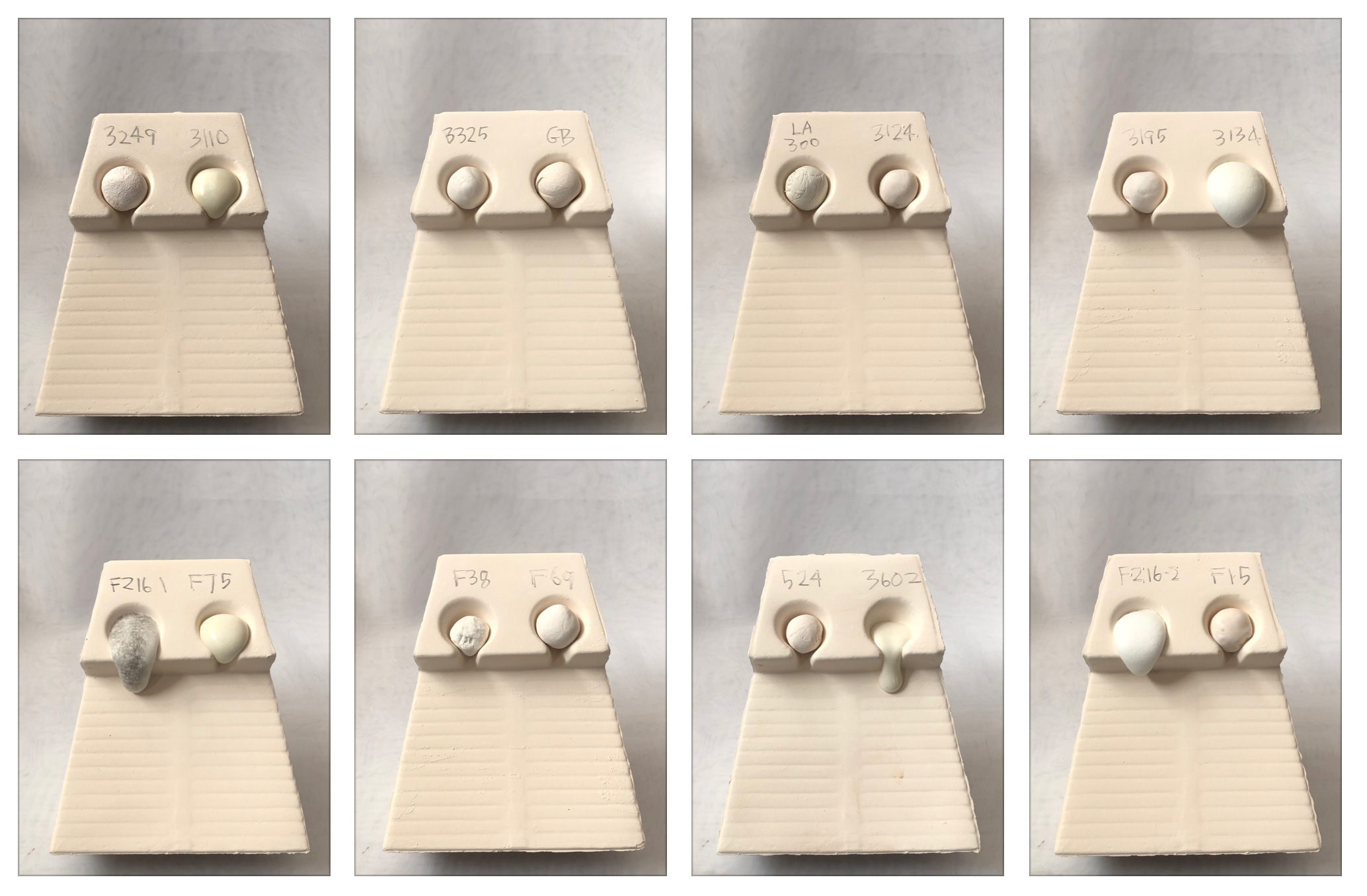
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1450F and held for 15 minutes. Frit 3134 is still expanding. 3602 is blasting out of the gate, taking the lead. F75 is starting to flow.
Melt fluidity comparison of frits - 1500F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1500F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are really starting to move. 3195, F38 and F15 are softening.
1700F Frit Melt-Off: Who is the winner? Not the lead bisilicate!

This picture has its own page with more detail, click here to see it.
These were 10g balls melted using our GBMF test. We fired at a temperature far lower than typical bisque, notice how many of them are already melting well! Frit 3602 is lead bisilicate. But it got "smoked" by the Fusion FZ-16 high-zinc, high-boron zero-alumina! Maybe you always thought lead was the best melter. That it produced the most transparent, crystal-clear glass. But that is not what we see here. That being said, notice the lead is not crazing but the FZ-16 is crazing badly, that is a problem for many applications using this frit, it relies on a high percentage of KNaO. Notice something else: Each frit has a distinctive melt fingerprint that makes it recognizable in tests like this. Want to get some of this frit for pottery? You can't, Fusion Ceramics doesn't want to handle retail sales of smaller quantities.
Frit Melt Fluidity Comparison - 1800F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1800F and held for 15 minutes (I already did firings from 1300F-1750F in 50 degree increments, all of them are visible in the parent project). Frit 3110, 3134, 3195, F75 have run all the way down. All of the frits have softened and melted slowly over a range of temperatures (hundreds of degrees). By contrast, Gerstley Borate, the only raw material here, suddenly melted and flowed right over the cliff (between 1600 and1650)! But not before Frit 3602 and FZ16 had done so earlier. Frit 3249 is just starting to soften but F69 (the Fusion Frits equivalent) is a little ahead of it. LA300 and Frit 3124 are starting also. F524, F38, F15 will all be over the end by the next firing. The melt surface tension is evident by the way in which the melts spread out or hold together.
Melt fluidity comparison of frits - 1550F
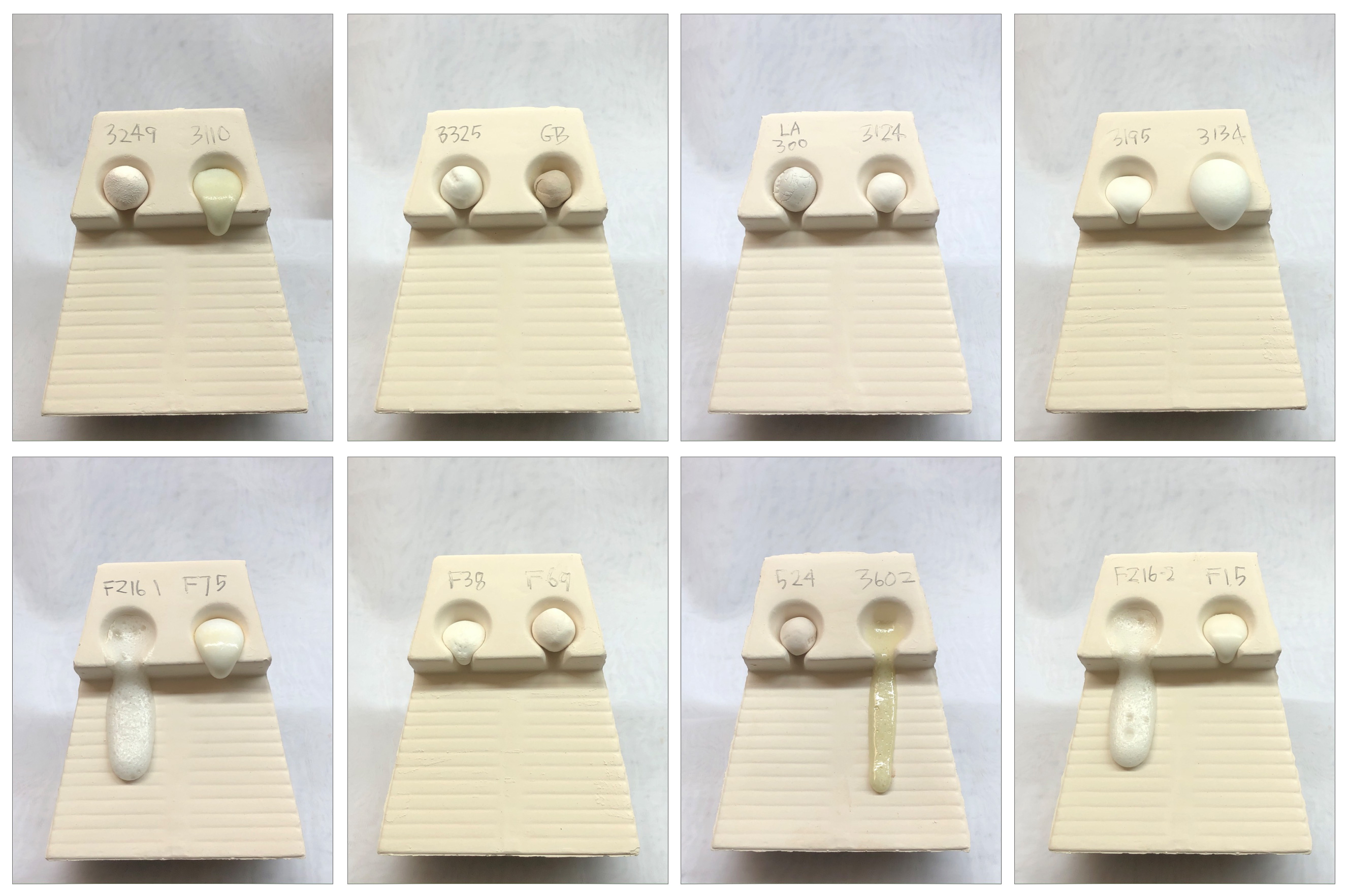
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1550F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are going to be off-ramp by next firing.
Melt fluidity comparison of frits - 1650F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1650F and held for 15 minutes. FZ16 has turned crystal clear and spread out across the runway (has low surface tension). Frit 3110 has so much surface tension that the flow can be lifted off the tester. Since 1600F Gerstley Borate has gone from unmelted to passing all the rest!
Melt fluidity comparison of frits - 1700F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1700F and held for 15 minutes. 3110 is finally starting to move. 3134 also (being full of bubbles). Gerstley Borate has turned almost transparent (because the Colemanite portion of it is now melting). 3195 is looking very well behaved compared to most others, forming a bubble free glass of high surface tension (F15 and F524 are starting to do the same).
Substitute Ferro Frit 3134, using glaze chemistry, in three glaze types

This picture has its own page with more detail, click here to see it.
Can't get frit 3134 for glaze recipes? Can you replace it with frit 3124? No, 3124 has five times the amount of Al2O3 (the second most important oxide in glazes) and half the amount of B2O3 (the main melter). This ten-minute video presents a glaze chemistry approach that is easier to do than you probably think. It deals with three different glaze recipe types lacking sufficient clay to suspend the slurry. Learn to source the needed oxides from two other Ferro frits, 3110 (or Fusion F-75) and 3195 (Fusion F-2), and end up with at least 15% kaolin in each. A unique approach is required in each situation. Two of the calculations produce improved slurry properties and one yields a recipe of significantly lower cost. If you have a recipe that needs this and need help please contact us.
Frit Melt Fluidity Comparison - 1850F

This picture has its own page with more detail, click here to see it.
These melt flow tests were fired at 350F/hr to 1850F and held for 15 minutes (I did firings at 50-degree increments across a wide range). It is amazing how active some frits are, even well below normal bisque temperatures! Frit 3110, Frit 3134, Frit 3195, Frit F-75 have all flowed all the way down for many previous temperatures. LA300 and Frit 3124 were just starting at 1800F, look at them now! Frit F-524 and Frit F-38 have gone from half-way at 1800F to water-falling over the end. Frit 3249 is still not out-of-the-gate but Frit F-69 (the Fusion Frits equivalent of 3249) is half-way. Note how the melt surface tension is evident by the way in which the melts spread out or hold together. By contrast, Gerstley Borate (labelled "GB"), the only raw material here, suddenly melted and flowed right over-the-cliff between 1600 and 1650! The best melter of all of them is high-boron high-zinc Frit FZ-16.
Links
| Materials |
Fusion Frit F-2
|
| Materials |
General Frit GF-115
|
| Materials |
Pemco Frit P-67
|
| Materials |
Frit
Frits are made by melting mixes of raw materials, quenching the melt in water, grinding the pebbles into a powder. Frits have chemistries raw materials cannot. |
| Materials |
PotteryCrafts Frit P3195
|
| Materials |
Potclays Frit 2269
|
| Materials |
Ceradel Frit 3195
|
| Materials |
Hommel Frit 399
|
| Typecodes |
Frit
A frit is the powdered form a man-made glass. Frits are premelted, then ground to a glass. They have tightly controlled chemistries, they are available for glazes of all types. |
| URLs |
https://digitalfire.com/4sight/datasheets/ferropotteryfrits2008.pdf
Ferro Pottery Frits 2008 |
| Recipes |
G1916Q - Low Fire Highly-Expansion-Adjustable Transparent
An expansion-adjustable cone 04 transparent glaze made using three common Ferro frits (low and high expansion), it produces an easy-to-use slurry. |
Data
| Co-efficient of Linear Expansion | 7.16 |
|---|---|
| Frit Melting Range (C) | 1500-1700F |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
