| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Colorant
In ceramics and pottery, colorants are added to glazes as metal oxides, metal-oxide-containing raw materials or as manufactured stains.
Key phrases linking here: colorants, colorant - Learn more
Details
Although colorants are added to bodies, most people think of them as materials that transform a colorless transparent or opaque glaze into a colored glaze. Colorants can be raw metal oxides (e.g. iron oxide, chrome oxide), metal-oxide-containing materials (e.g. rutile), or man-made powders that are smelted from metal oxide and stabilizer mixes (stains). Potters and smaller companies often use raw colorants and color-containing raw materials whereas industrial manufacturers employ stains. Unlike stains which are prefired, the color of raw colorant powders often bears no resemblance to the color they will produce in a glaze. Raw metal oxide powders are often available as carbonates or oxides (other forms are typically available, e.g. sulfates, but they are not usually used in ceramics for solubility or other issues). Carbonate colors can gas during firing (since the carbonates decompose and volatilize). This can be a source of glaze defects when the gassing happens after the glaze has begun to melt (copper carbonate and manganese dioxide are known for this issue). Color-containing raw materials, like rutile, often vary in their chemistry and mineralogy and are the least reliable option for coloring glazes. Not all metal oxides produce color (e.g. tin, titanium, zircon), but they can affect existing color, stabilize it or opacify the glass.
In glazes, color is a matter of chemistry: The color produced depends on the oxide make-up of the host glaze and of the mix of colorants added. The same metal oxide can participate in many color systems. Some colorants produce the same color across a wide range of host glazes (e.g. cobalt), others are very sensitive to the presence or absence of specific helper or hostile oxides (e.g. chrome-tin combinations). Colors are the most vibrant in transparent glazes where there is depth (in opaque glazes they tend to produce pastel shades). Some colors are potent, 1% can produce a strong color. Others are weak and 10% or more may be needed.
Colorants affect fired properties of glazes (according to their concentration) like any other material (e.g. thermal expansion, melting temperature, gloss). For example, some are powerful fluxes, others are very refractory. However, the specifics of how, and the degree to which they affect the fired glass are not nearly as well known as for other common ceramic oxides. Generally, technicians know how much of a colorant is needed to achieve the shade they want and they know what chemistry the host glaze needs to have to maximize the efficiency of the color (since colorants are the most expensive of all ceramic materials). Beyond that, it is common to not include the colorant when calculating the oxide chemistry of a glaze. People rationalize and control the fired properties of the colorless base glaze in terms of the chemistry while they rationalize the contribution of the colorants at the recipe level.
Metal oxides (e.g. iron oxide, cobalt oxide, chrome oxide) can be completely safe or have considerable toxicity. The danger can be in handling the raw powders, breathing their dust, breathing fumes generated during firing, or eating from glazes saturated by them (where metals can leach into food and drink). Generally, food surface glazes should have low percentages of raw metal oxides (a transparent or white liner glaze is better). If color is important, stains should be used instead (unless the colorants is known to be safe e.g. iron oxide). Sometimes a colorant-containing glaze can be stable and durable as-is, but when a percentage of another metal oxide is added (e.g. copper oxide), the glaze as-a-whole becomes more leachable. Special caution should be exercised with glaze recipes found online. Flashy pictures can look good, but often the visual effect is especially dependent on the glaze being over or under melted or being unbalanced in its chemistry. It is better to have a base glaze that your know works well on your clay and is safe (transparent matte or glossy) and add colorants, opacifiers and variegators to it.
Related Information
Iron oxide powder is available in many colors. Here are three.

This picture has its own page with more detail, click here to see it.
How can there be so many colors? Because iron and oxygen can combine in many ways. In ceramics we know Fe2O3 as red iron and Fe3O4 as black iron (the latter being the more concentrated form). But would you believe there are 6 others (one is Fe13O19!). And four phases of Fe2O3. Plus more iron hydroxides (yellow iron is Fe(OH)3).
Chrome oxide powder

This picture has its own page with more detail, click here to see it.
A problem with brilliantly colored fluid-melt glazes: Micro-crystals

This picture has its own page with more detail, click here to see it.
Crystallization (also called devritrification). You can see the tiny crystals on the surface of this copper stained cone 6 glaze (G3806C). The preferred orientation of metallic oxides is crystalline. When kilns cool quickly there is simply not enough time for oxides in an average glaze to organize themselves in the preferred way and therefore crystals do not grow. But if the glaze has a fluid melt and it cools slowly through the temperature at which the crystals like to form, they will. There is another issue here also: There are tiny dimples in the surface. This is because copper carbonate was used here instead of copper oxide. During firing, it generates carbon dioxide (because it is a carbonate) that bubbles out of the melt, leaving behind dimples that may or may not heal during cooling.
MgO can destroy the rutile blue variegation effect

This picture has its own page with more detail, click here to see it.
The rutile blue variegation effect is fragile. It needs the right melt fluidity, the right chemistry and the right cooling (during firing). This is Alberta Slip GA6C recipe on the right (normal), the glaze melt flows well due to a 20% addition of Ferro Frit 3134 (a very low melting glass). On the left Boraq has been used as the flux (it is a calcium borate and also melts low, but not as low as the frit). It also contains significant MgO. These two factors have destroyed the rutile blue effect!
How do metal oxides compare in their degrees of melting?

This picture has its own page with more detail, click here to see it.
These metal oxides have been mixed with 50% Ferro frit 3134 and fired to cone 6 oxidation. Chrome and rutile have not melted, copper and cobalt are extremely active melters, frothing and boiling. Cobalt and copper have crystallized during cooling. Manganese has formed an iridescent glass.
Cone 10 Reduction, the home of an amazing oxide: Iron

This picture has its own page with more detail, click here to see it.
It is a powerful glaze flux, variegator and crystalizer, a colorant of many characters in bodies and glazes and a specking agent like no other. And it is safe and cheap!
Mason stains in the G2926B base glaze at cone 6

This picture has its own page with more detail, click here to see it.
This glaze, G2926B, is our main glossy base recipe. Stains are a much better choice for coloring it than raw metal oxides. Other than the great colors they produce here, there are a number of things worth noticing. Stains are potent; the percentages needed are normally much less than for metal oxides. Staining a transparent glaze produces a transparent color, it is more intense where the laydown is thicker - this is often desirable in highlighting contours and designs. For pastel shades, add an opacifier (e.g. 5-10% Zircopax, more stain might be needed to maintain the color intensity). The chrome-tin maroon 6006 does not develop well in this base (alternatives are G2916F or G1214M). The 6020 manganese alumina pink is also not developing here (it is a body stain). Caution is required with inclusion stains (like #6021). Bubbling, as is happening here, is common - this can be mitigated by adding 1-2% Zircopax. And it’s easy to turn any of these into brushing or dipping glazes.
Maroon and white mug before and after firing: What a difference!

This picture has its own page with more detail, click here to see it.
The outer glaze is Ravenscrag GR6-E Raspberry, the bright maroon color is a product of the surprising interaction between the 0.5% chrome oxide and 7.5% tin oxide present. That small amount of chrome is only enough to give the raw powder a slight greenish hue, hardly different than the clear liner glaze. While this color mechanism appears to be effective, it is delicate. A maroon stain is actually a better choice. It would fire more consistent would be less hazardous to use. And the raw glaze will be the same color as the fired one!
Example of a data sheet for Copper Carbonate
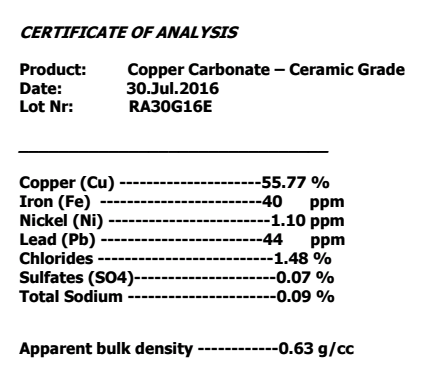
This picture has its own page with more detail, click here to see it.
Notice it does not quote the amount of CuCO3, just Cu metal. It also does not quote LOI percentage (weight loss on ignition, it will be more than 25%). Theoretical copper carbonate is 71.94% CuO (sourced by a mix of copper carbonate and carbonate hydroxide). CuO is 79.9% copper and 20.1% oxygen. Thus, we would expect Cu metal to be 57.5% (in a theoretical material). Since this example has impurities it is a little less, 55.8%.
Links
| Glossary |
Encapsulated Stain
This is a type of stain manufacture that enables the use of metal oxides (like cadmium) under temperature conditions in which they would normally fail. |
| Glossary |
Opacifier
Glaze opacity refers to the degree to which it is opaque. Opacifiers are powders added to transparent ceramic glazes to make them opaque. |
| Glossary |
Transparent Glazes
Every glossy ceramic glaze is actually a base transparent with added opacifiers and colorants. So understand how to make a good transparent, then build other glazes on it. |
| Glossary |
Limit Formula
A way of establishing guideline for each oxide in the chemistry for different ceramic glaze types. Understanding the roles of each oxide and the limits of this approach are a key to effectively using these guidelines. |
| Glossary |
Ceramic Stain
Ceramic stains are manufactured powders. They are used as an alternative to employing metal oxide powders and have many advantages. |
| Materials |
Stain
|
| Properties | Glaze Color |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
