| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Ball Clay
Description: Highly Plastic Fine Particle Clay
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 0.30% | 0.02 | |
| K2O | 0.90% | 0.04 | |
| MgO | 0.30% | 0.03 | |
| Na2O | 0.40% | 0.03 | |
| TiO2 | 1.00% | 0.05 | |
| Al2O3 | 25.00% | 1.00 | |
| SiO2 | 59.00% | 4.01 | |
| Fe2O3 | 1.00% | 0.03 | |
| LOI | 12.00% | n/a | |
| Oxide Weight | 358.63 | ||
| Formula Weight | 407.54 | ||
Notes
The term 'ball' traces to historic mining in England where large chunks of the clay were cut from the bank in ball shapes for transport to processing. There are hundreds of different ball clays available. Potentially they should vary widely in plasticity, particle size, raw color, and drying properties. But in practice, most are remarkably similar. A typical ball clay powder is light grey (from lignite) or cream color and fires to a buff or cream (with some soluble salt deposits on the fired surface). Ball clays tend to be quite refractory (PCE 28-34) and some less processed deposits are sold as fireclays. Ball clay is not a clay mineral in itself, but contains other minerals, primarily kaolinite (but also montmorillonite, halloysite, and illite). Mica and quartz are also present in substantial amounts (e.g. 10-20% for Tennessee ball clays).
Ball clays are very plastic and much finer-grained than kaolins. They are easily slaked in water when dry. Few people fully appreciate how 'sticky' and plastic these materials are until they mix some with water and work with it pure. The fine particle size also makes them impermeable to the passage of water (a small test bar can take a very long time to dry).
While potters only buy ball clay as a powder, industrial users have access to the material in many forms (shredded, noodled and partially deflocculated, chopped filter press cake, vacuum pugged extrusion, crumble with about 10% moisture). These forms enable a more consistent supply and alot of flexibility, especially when making casting slips (since ball clays are the most difficult to disperse materials in the bodies).
They are typically unvitrified at cone 10. There are a wide range of ball clays used in traditional ceramic manufacture in North American and they have surprisingly similar firing characteristics (maturity and color). In one test we made 50:50 ball clay:feldspar mixes of 8 different common ball clays (from a range of suppliers), from cone 6 to 10 all had the same porosity and fired appearance.
Ball clays are used in ceramic bodies (porcelains, stonewares and earthenwares, casting slips, pressing bodies) because of their plastic nature combined with high firing temperature. Ball clays have very high dry shrinkage combined with high green strength and slow drying. Were it not for their iron and coal impurities, ball clays would be ideal ceramic materials. However, in practical terms, they are employed to achieve desired plasticity, but are minimized to reduce the detrimental effect on fired whiteness and drying properties.
A common starting recipe for a high temperature general purpose porcelain (as is used in electrical porcelain or extruded pottery porcelain is 25% each of ball clay, kaolin, feldspar and silica). The ball clay:kaolin mix can be altered to change body plasticity without significantly affecting the maturing temperature.
In North America, most commercial ball clays are mined in the southeastern US. Ball clay deposits are common and were laid by the action of slow moving water with an acidity that tended to flocculate and settle the clay. It is common to find lignite associated with ball clay, and this accounts for the almost grey appearance of many varieties (when wet).
It is difficult to compare their plasticities by quantitative tests because pure samples are difficult to mix and form (they are incredibly sticky) and crack badly during drying. Thus, it is common to mix ball clay and silica 50:50 and compare dry shrinkage, dry strength, soluble salts, fired color. Another technique to mix raw:calcine 50:50. In our testing of many ball clays, drying shrinkage and water of plasticity it very similar (those intended for casting have a little lower drying shrinkage, but they also fire very similar). In general it may be said that English ball clays tend to have a higher dry strength (and thus drying shrinkage) than American ones, Kentucky ball clays have the lowest carbonaceous matter, English ones vitrify lower, Tennessee ones fire whitest.
Although some ball clays resist deflocculation because of hostile soluble impurities, most deflocculate very well with sodium silicate and other equivalent dispersants. A wide range of ball clay slurries and slips are used in casting at all temperature ranges. One common recipe uses a simple 50:50 ball clay:talc mix (in the hobby casting market). The same mix is also dry pressed in the tile industry, and extruded for jiggering and wet processing in artware.
The refractories industry is a large user of ball clay. Common refractory materials lack plasticity and ball clay is used to help in forming and shape retention and to impart dry strength. The abrasives industry likewise uses it to bond aggregates until firing fuses the mass.
Engobes in the tile and brick industries are suspended, hardened, and adjusted to match body shrinkage by the addition of ball clay. Many pottery glazes contain ball clay to help suspend and harden them and control their shrinkage during drying (although some technicians prefer cleaner kaolins for this, claiming they gel the slurry better and prevent drip-drip during drying). It makes a big difference what ball clays are used, some produce a syrupy mess that settles while others produce a beautifully suspended creamy slurry. In North America, Old Hickory #5 and Old Hickory No. 1 Glaze ball clays work well in glazes. 10-15% ball clay should be enough to suspend a glaze, if there is any less add 1-2% bentonite. Recipes with 20% or more ball clay risk shrinking and cracking during drying.
If the iron or lignite content of ball clay is a problem, it is common to employ bentonite to reduce the ball clay requirements (5% bentonite can provide as much improvement in plasticity and dry strength as 25% ball clay). However, care is recommended to make sure a fine grade of bentonite is used to avoid fired specks (bentonite also burns darker).
Unlike a kaolin, it is difficult to establish a generic or theoretical analysis, we have provided one for a typical Kentucky ball clay.
If you use ball clay in your production there is good reason to be doing routine quality control to make sure it is remaining consistent. Ball clays are likely the most variable material you will have to deal with. They can sometimes have particulate impurities (especially lignite) and exhibit differences in soluble salts content, drying shrinkage, drying performance, fired maturity, fired color and behavior in slurries. Consider the SHAB test (see link below).
Adequate quartz content is an important factor in porcelain and whiteware bodies (it is an important structural element in the fired matrix and it is needed to prevent glaze crazing). Since ball clays contain quartz, it is possible to use less raw silica (quartz) powder in the recipe if ball clay percentages are high. Of course, the quartz grains in the ball clay are finer, so they will dissolve into the feldspar glass more readily.
Related Information

This picture has its own page with more detail, click here to see it.
Ball clay and kaolin test bars side-by-side fired from cone 9-11 oxidation and 10 reduction.
The difference in fired character between kaolin and ball clay at cone 10R

This picture has its own page with more detail, click here to see it.
The top one is EP Kaolin, the bottom one is Old Hickory M23 Ball Clay (these materials are typical of their respective types). These materials have low alkali contents (especially the kaolin), this lack of flux means they are theoretically highly refractory mixes of SiO2 and Al2O3. It is interesting that, although the kaolin has a much larger ultimate particle size, it is shrinking much more (23% total vs. 14%). This is even more unexpected since, given that it has a lower drying shrinkage, and should be more refractory. Further, the kaolin has a porosity of 0.5% vs. the ball clay's 1.5%. The kaolin should theoretically have a much higher porosity? What is more, both of these values are unexpectedly low. This can partly be explained by the particle packing achieved because of the fine particle size. Despite these observations, their refractory nature is ultimately proven by the fact that both of these can be fired much higher and they will only slowly densify toward zero porosity.
OM4 Ball clay fired from cone 10R (top), 10 down to 4 (downward)

This picture has its own page with more detail, click here to see it.
Ball clays are normally refractory, none of these are vitrified to any extent. The cone 10R bar is yellow because it is stained by the soluble salts present in the material. These are very typical of what most ball clays look like.
Four North American ball clays fired at high temperature

This picture has its own page with more detail, click here to see it.
The top bars went to cone 10R, the others are cone 11 and 10 oxidation (downward from top). They are Gleason, Spinks Blend, OM#4 and NP Blend. It is amazing now similar different ball clays fire in the kiln. Clearly, soluble salts are an issue with all of them (the brownish scum). These bars are much cleaner on the backsides (since the solubles were left on the surface on the fronts during drying). The drying shrinkages, plasticities and fired maturities are also all remarkably similar.
Ball clay vs. Kaolin porcelain at cone 6

This picture has its own page with more detail, click here to see it.
Left: A porcelain that is plasticized using only ball clays (Spinx Gleason and Old Hickory #5). Right: Only kaolin (in this case Grolleg). Kaolins are much less plastic so bentonite (e.g. 2-5%) is typically needed to get good plasticity. The color can be alot whiter using a clean kaolin, but there are down sides. Kaolins have double the LOI of ball clays, so there are more gasses that potentially need to bubble up through the glaze (ball clay porcelains can produce brilliantly glassy and clean results in transparent glazes even at fast fire, while pure kaolins can produce tiny dimples in the glaze surface if firings are not soaked long enough). Kaolins plasticized by bentonite often do not dry as well as ball clays even though the drying shrinkage is usually less. Strangely, even though ball clays are so much harder and stronger in the dry state, a porcelain made using only ball clays often still needs some bentonite. If you do not need the very whitest result, it seems that a hibrid using both is still the best general purpose, low cost answer.
Cleanest kaolin porcelain vs. ball-clay-only porcelain!

This picture has its own page with more detail, click here to see it.
These cone 6 clear-glazed porcelains demonstrate just how white you can make a porcelain if you use white burning kaolins and bentonites instead of ball clays. Both contain about 40% clay. The one on the left employs New Zealand kaolin and Veegum plasticizer, the one on the right Kentucky ball clays (among the whitest of ball clays in North America) and standard bentonite. Both are zero porosity. The glaze surface is a little more flawless on the right one (possibly because ball clays have a lower LOI than kaolins).
Ball clay powders are minus 200 mesh. Right? Wrong!

This picture has its own page with more detail, click here to see it.
These are the oversize particles (from the 79, 100, 140 and 200 mesh sieves) from 100 grams of a commercial Gleason ball clay. They have been fired to cone 8 oxidation. There is 1.5 grams total, this is within the limits stated on their data sheet even though the material is sold as 200 mesh grade. Firing the samples shows whether the particles contain iron that will produce specking in porcelains and whiteware. In this case there are a few. We do this test on many materials and this is typical of what we see.
Cone 6 kaolin porcelain verses ball clay porcelain.

This picture has its own page with more detail, click here to see it.
Typical porcelains are made using clay (for workability), feldspar (for fired maturity) and silica (for structural integrity and glaze fit). These cone 6 test bars demonstrate the fired color difference between using kaolin (top) and ball clay (bottom). The top one employs #6 Tile super plastic kaolin, but even with this it still needs a 3% bentonite addition for plasticity. The bottom one uses Old Hickory #5 and M23, these are very clean ball clays but still nowhere near the whiteness of kaolins. Plus, 1% bentonite was still needed to get adequate plasticity for throwing. Which is better? For workability and drying, the bottom one is much better. For fired appearance, the top one.
A refractory ball clay vs a vitreous ball clay

This picture has its own page with more detail, click here to see it.
L3835A (KT1-4 ball clay) and L3835F (Spinks Blend ball clay), each mixed 65:35 ball clay and nepheline syenite. This mix produces a zero porosity body by cone 5.
A Super Plastic Stoneware Made With Two Materials
This mix showcases stoneware's advantages over porcelain

This picture has its own page with more detail, click here to see it.
Left: 65% #6Tile kaolin and 35% nepheline syenite. Although it has great plasticity and fires white, it crazes the glaze and has 1% fired porosity (using the SHAB test).
Right: 65% M23 Old Hickory ball clay (similar to OM#4) and 35% nepheline syenite (feldspar would also work). The glaze fits, the body fires very dense (zero porosity) and plasticity is fantastic!
The body on the left needs a 20% silica addition (to stop the crazing) and more nepheline (to reduce porosity to porcelain levels). The remaining 40% kaolin will not be nearly enough for a workable plasticity (so bentonite will be needed). The body on the right does not need fixing; it works as is. Many feel that stoneware body recipes must be built on a feldspar-containing base clay having pottery plasticity (adding ball clay, kaolin, feldspar and silica and ending up with needlessly complicated recipes). But, ball clay is a base, all it needs is a non-plastic filler (to cut plasticity and therefore drying shrinkage) and a flux to vitrify it - feldspar or nepheline fills both roles.
Of course, this stoneware does not fire as white. But do you need white? Glazes will fire brightly colored on this surface. And, cleaner ball clays are available in many areas (even ball clays intended for casting will be plastic enough). It is also worth considering the use of a white engobe.
What would happen if you made a clay body from 50:50 kaolin and ball clay?

This picture has its own page with more detail, click here to see it.
It would craze glazes! Really badly (this is fired at cone 6). One might think that there is adequate quartz in this high of a percentage of ball clay to at least minimize crazing, even causing shivering. At cone 10 oxidation this has about 5% porosity (the ball clay contributes enough iron that porosity drops to 2% in reduction). While an addition of feldspar would cut this somewhat, only more silica will increase thermal expansion enough to put the squeeze on glazes to prevent crazing like this.
Even heavy soluble salts may not have a significant affect on the glaze

This picture has its own page with more detail, click here to see it.
This is a ball clay. They are known to produce this type of soluble salts when fired at high temperature reduction (the inner salt-free section is such because that part of the tile was covered during drying, so the soluble salts from there had to migrate to the outer exposed edge). If soluble salts fire to a glassy surface they can affect the overlying glaze. But in this case they are not and have a minimal effect.
Health warning phrases on a bag of ball clay

This picture has its own page with more detail, click here to see it.
The ball clay you use to suspend your glaze is important!
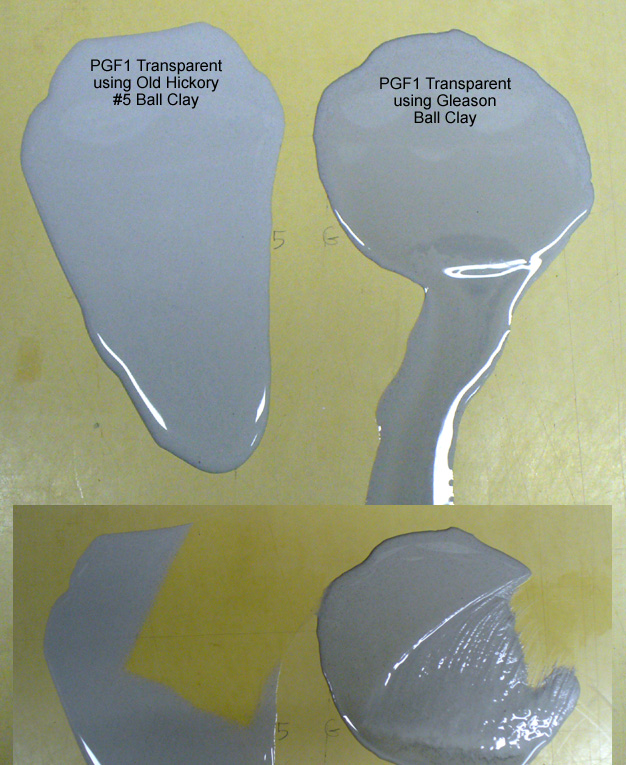
This picture has its own page with more detail, click here to see it.
I poured 4 teaspoons of two glazes onto a non-absorbent butcher’s board and let them sit for a minute, then inclined the board. The one on the right employs Gleason Ball clay, the left one has Old Hickory #5 ball clay. Neither has any rheology modifier additions. The one on the right has settled and, on incline of the board, the watery upper is running off. The other has gelled and the whole thing is running downward slowly. Below that you can see where I have begun to sponge them off, the one on the right is sticky. The most amazing thing about this: This difference appears despite the fact that there is only 7% ball clay in this heavily fritted recipe.
Can we ball mill a clay and make it more colloidal? Yes.

This picture has its own page with more detail, click here to see it.
This 1000 ml 24 hour sedimentation test compares Plainsman A2 ball clay ground to 10 mesh (left) with that same material ball milled for an hour (right). The 10 mesh designation is a little misleading, those are agglomerates. When it is put into water many of those particles break down releasing the ultimates and it does suspend fairly well. But after 24 hours, not only has it settled completely from the upper section but there is a heavy sediment on the bottom. But with the milled material it has only settled slightly and there is no sediment on the bottom. Clearly, using an industrial attrition ball mill this material could be made completely colloidal.
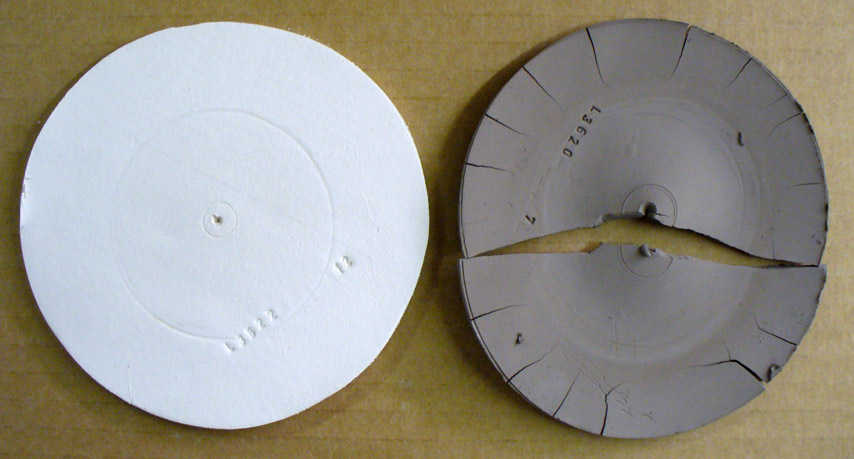
High drying shrinkage of Plainsman A2 ball clay (DFAC disk)

This picture has its own page with more detail, click here to see it.
This DFAC test disk shows the incredible drying shrinkage that a ball clay can have. Obviously if too much of this is employed in a body recipe one can expect it to put stress on the body during drying. Nevertheless, the dry strength of this material far exceeds that of a kaolin and when used judiciously it can really improve the working properties of a body giving the added benefit of extra dry strength.
How a kaolin and ball clay compare in a dry performance test
This picture has its own page with more detail, click here to see it.
These are DFAC drying performance disks of a large-particle kaolin (OptiKast) and a ball clay (Plainsman A2). This test reveals a clay's response to uneven drying (these disks are dried with the center portion covered). The kaolin feels smoother yet its ultimate particles are ten to one hundred times bigger than a typical ball clay. Thus it shrinks much less. The ball clay has dramatically lower water permeability, water from the center protected portion resists migration to the outer edge during drying. When the inner section finally dried the outer was already rigid so it split the disk in two and pulled all the edge cracks. Most ball clays shrink more and crack worse than this (cracks concentric to the center also appear). So why use ball clay? This kaolin is so lacking in plasticity it was barely possible to even make this disk. And it is so weak that it can easily break just by handling it. Still, it is useful to make casting bodies. But the ball clay, when used as a percentage of a body mix, can produce highly plastic bodies than can be dried without trouble if done evenly.
Carbon burnout in a ball clay

This picture has its own page with more detail, click here to see it.
A broken test bar of ball clay fired to cone 10 reduction. Notice the black carbon core. Ball clays commonly contain carbon, many have a noticeable grey color in the raw state because of this. Notice it has not burned out despite the fact that the clay itself is still fairly porous, the firing was slow and the temperature reached was high. Ball clay typically does not comprise more than 30% of a body recipe so its opportunity to burn away is sufficient. However some specialized bodies have a much higher percentage.
Watch out for iron particles in ball clays

This picture has its own page with more detail, click here to see it.
These are the oversize particles (from the 70, 100, 140 and 200 mesh sieves) from 100 grams of a commercial ball clay. They have been fired to cone 10 reduction. As you can see, this material is a potential cause of specking, especially in porcelain bodies. It is not only wise to check for oversize particles in clays, but firing these particles will reveaal if they contain iron. A 200 mesh screen would be a good start for this test, it would catch all of these.
What if you just cannot solve a pinhole problem?

This picture has its own page with more detail, click here to see it.
Pinholing on the inside of a cone 6 whiteware bowl. This is glaze G2926B. The cause is likely a combination of thick glaze layer and gas-producing particles in the body. Bodies containing ball clays and bentonites often have particles in the +150 and even +100 mesh sizes. The presence of such particles is often sporadic, thus it is possible to produce defect-free ware for a time. But at some point problems will be encountered. Companies in large production need to have fast firing schedules, so they either have to filter press or wet process these bodies to remove the particles. Or, they need to switch to more expensive bodies containing only kaolins and highly processed plasticizers. But potters have the freedom to use drop-and-hold or slow-cool firing schedules, that single factor can solve even serious pinholing issues.
Will soil testing help you assess a wild clay for pottery?

This picture has its own page with more detail, click here to see it.
No, soil testing is not helpful. Soils normally contain clay but it is so diluted with sand, rocks, silt and organics that overall plasticity is just a dot on a graph - not even close to what modelling or throwing clays exhibit. Pottery clays easily hold a shape and can be adjusted to a new shape without splitting. They dry slowly with substantial shrinkage. Highly plastic clays need more water to achieve working consistency, silty non-plastic ones need less (typical pottery clays need 18-23%). The reports shown here are typical for soils. But almost nothing here would look familiar to a potter.
-The Shrinkage Limit (SL) is the water content where further loss of moisture will not result in any more volume reduction.
-The Plastic Limit (PL) is minimum water content at which a soil is considered to behave in a ‘plastic’ manner, i.e. is capable of being moulded.
-The Liquid Limit (LL) is the maximum water content a silt or clay can have before becoming a liquid, i.e. turning into mud.
-The Plasticity Index (PI) is the range of moisture contents where the silt or clay remains plastic (PI = LL – PL).
Potters don't care about the amount of water needed, they care about how plastic the clay is once enough water has been added to get the right stiffness.
Links
| Materials |
Bentonite
Bentonite can make a clay body instantly plastic, only 2-3% can have a big effect. It also suspends slurries so they don't settle out and slows down drying. |
| Materials |
BLUE-CLAY
|
| Materials |
44-B Ball Clay
|
| Materials |
49'er Ball Clay
|
| Materials |
5-S Ball Clay
|
| Materials |
54-S Ball Clay
|
| Materials |
56-S Ball Clay
|
| Materials |
Anglo No. 1 Ball Clay
|
| Materials |
No. 1 Ball Clay
|
| Materials |
Bell Dark Ball Clay
|
| Materials |
BKS/L Ball Clay
|
| Materials |
C & C Ball Clay
|
| Materials |
Caroso Ball Clay
|
| Materials |
Caroso FC Ball Clay
|
| Materials |
CDI Ball Clay
|
| Materials |
CDL Ball Clay
|
| Materials |
Coppen Light Clay
|
| Materials |
Crenshaw Ball Clay
|
| Materials |
Cresan C Ball Clay
|
| Materials |
Dresden M Ball Clay
|
| Materials |
Eobb/y Ball Clay
|
| Materials |
Esva Ball Clay
|
| Materials |
EWVA Ball Clay
|
| Materials |
F-2 Ball Clay
|
| Materials |
Fayles Blue Ball
|
| Materials |
Old Hickory FC340 Ball Clay
|
| Materials |
FX Ball Clay
|
| Materials |
Gresan C Ball Clay
|
| Materials |
HB-11 Ball Clay
|
| Materials |
HTP Ball Clay
|
| Materials |
Huntingdon Ball Clay
|
| Materials |
HVA/ND Ball Clay
|
| Materials |
HVA/PM Ball Clay
|
| Materials |
HVA/R Ball Clay
|
| Materials |
Hymod AT Ball Clay
|
| Materials |
Hyplas 71 Ball Clay
|
| Materials |
Imperial Ball Clay
|
| Materials |
J-2 Ball Clay
|
| Materials |
Jackson Ball Clay
|
| Materials |
Starcast Ball Clay
|
| Materials |
Stratton Ball Clay
|
| Materials |
SX Blue Ball Clay
|
| Materials |
TA Ball Clay
|
| Materials |
Taylor Blend
|
| Materials |
TB-1 Ball Clay
|
| Materials |
Tennessee #1 SGP
|
| Materials |
Tennessee #10 Ball Clay
|
| Materials |
Tennessee #5 Ball
|
| Materials |
Tennessee #6 Bond
|
| Materials |
Tennessee #9 Ball Clay
|
| Materials |
Texas GK Ball Clay
|
| Materials |
Thomas Ball Clay
|
| Materials |
TI-21 Ball Clay
|
| Materials |
Todd Dark Ball Clay
|
| Materials |
Todd Light Ball Clay
|
| Materials |
TWVD Ball Clay
|
| Materials |
Victoria Ball Clay
|
| Materials |
Volunteer Ball Clay
|
| Materials |
Yankee Ball Clay
|
| Materials |
CP-7 Ball Clay
|
| Materials |
Hyplas 64 Ball Clay
|
| Materials |
J-4 Ball Clay
|
| Materials |
HA-5 Ball Clay
|
| Materials |
MB Ball Clay
|
| Materials |
OSML Ball Clay
|
| Materials |
SB Ball Clay
|
| Materials |
CTS Ball Clay
|
| Materials |
JASS Ball Clay
|
| Materials |
3380 Ball Clay
|
| Materials |
RG1 Ball Clay
|
| Materials |
Gizmo Ball Clay
|
| Materials |
Kentucky #12
|
| Materials |
Kentucky #6
|
| Materials |
Royal Ball Clay
|
| Materials |
Cooley No. 2 Ball Clay
|
| Materials |
Kaolin
The purest of all clays in nature. Kaolins are used in porcelains and stonewares to impart whiteness, in glazes to supply Al2O3 and to suspend slurries. |
| Materials |
Ball Clay FX (CA1)
|
| Materials |
Ball Clay R
|
| Materials |
No. 5 Ball Clay
|
| Hazards |
Ball Clay
Most stoneware and whiteware clay bodies depend on ball clay for their plasticity (50%+ is common). It is also found in lower percentages in porcelains. It is sticky, plastic, fine particled and contains some free quartz. |
| Hazards |
Dioxins in Clays
DIOXINS in Clays used in ceramics, what are the dangers? |
| Hazards |
Dealing With Dust in Ceramics
A checklist for changes and additions to your tools and equipment and suggestions for habit you need to develop to control dust in your workplace. |
| Typecodes |
Ball Clay
Ball clays are abundant and very plastic and are used in all types of plastic forming bodies. They are not as white-burning or refractory as kaolins but lower in iron and fluxes than bentonites. |
| Typecodes |
Generic Material
Generic materials are those with no brand name. Normally they are theoretical, the chemistry portrays what a specimen would be if it had no contamination. Generic materials are helpful in educational situations where students need to study material theory (later they graduate to dealing with real world materials). They are also helpful where the chemistry of an actual material is not known. Often the accuracy of calculations is sufficient using generic materials. |
| Typecodes |
Ball Clay
Ball clays are abundant and very plastic and are used in all types of plastic forming bodies. They are not as white-burning or refractory as kaolins but lower in iron and fluxes than bentonites. |
| Oxides | Al2O3 - Aluminum Oxide, Alumina |
| Oxides | C - Carbon |
| Glossary |
Plasticity
Plasticity (in ceramics) is a property exhibited by soft clay. Force exerted effects a change in shape and the clay exhibits no tendency to return to the old shape. Elasticity is the opposite. |
| Glossary |
Clay
What is clay? How is it different than dirt? For ceramics, the answer lies on the microscopic level with the particle shape, size and how the surfaces interact with water. |
| Glossary |
Deflocculation
Deflocculation is the magic behind the ceramic casting process, it enables slurries having impossibly low water contents and ware having amazingly low drying shrinkage |
| Glossary |
Stoneware
To potters, stonewares are simply high temperature, non-white bodies fired to sufficient density to make functional ware that is strong and durable. |
| Glossary |
Permeability
In ceramics, the permeability of clay slurries and plastics determines the rate as which water can move through the matrix |
| Tests |
Shrinkage/Absorption Test
SHAB Shrinkage and absorption test procedure for plastic clay bodies and materials |
| Tests |
Sieve Analysis 35-325 Wet
A measure of particle size distribution by washing a powdered or slaked sample through a series of successively finer sieves |
| Articles |
Formulating a Porcelain
The principles behind formulating a porcelain are quite simple. You just need to know the purpose of each material, a starting recipe and a testing regimen. |
| URLs |
http://www.oldhickoryclay.com/pdf_files/Data_Ball_Clays.pdf
Old Hickory Clays conference presentation on ball clays (educational) |
Mechanisms
| Body Plasticity | Ball clay is the main plastic material used in clay bodies of all types. It is much more plastic than kaolin but also has much higher dry shrinkage and higher iron content. A typical white high temperature stoneware is often about 25% each of kaolin, ball clay, feldspar and silica. |
|---|---|
| Glaze Suspender | Ball clay is a fine particled clay that is universally used in glazes for suspension. If 15-20% is present no other suspender should be needed. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
