| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Gillespie Borate
Alternate Names: Gilespie Borate
Description: Gerstley Borate Substitute
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| SiO2 | 11.80% | 0.34 | |
| B2O3 | 24.50% | 0.62 | |
| Al2O3 | 1.70% | 0.03 | |
| SrO | 0.45% | 0.01 | |
| Na2O | 3.77% | 0.11 | |
| MgO | 3.90% | 0.17 | |
| CaO | 23.00% | 0.72 | |
| LOI | 30.90% | n/a | |
| Oxide Weight | 120.86 | ||
| Formula Weight | 174.90 | ||
Notes
Since August 2023 we have received multi-pallet shipments and tested it side-by-side with vintage Gerstley Borate (see below).
This substitute became available during the early 2000s and won the attention of many Gerstley Borate (GB) users (it is still available in 2023 and they state readiness to take the market from GB). With Gillespie Borate, they claim to achieve chemistry, mineralogy and physical properties very similar to GB. They went well beyond what other companies did in explaining their research, development and testing (however, their QuickFacts web page is now gone). Of course, it is assumed this material does not turn glaze slurries into a bucket of jelly the way GB did.
Here is a summary of some of the claims they were making:
"Gillespie Borate is a blended borate mineral for use in glaze formulas replacing Gerstley Borate on a pound-for-pound basis. It has better consistency, gives increased glaze surface smoothness, reduces crawling and pinholes and has fewer impurities so glazes and colors are brighter."
By virtue of having the same mineral profile, their product variegated glaze surfaces the same way. They did x-ray diffraction and physical tests to fully characterize GB and identified three key characteristics: extended particle size distribution including a colloid fraction, the oxide analysis, and firing properties that promote variegation. They identified immiscibility and solubility as key characteristics, the former generating the characteristic variegated texture and the latter the mottled glaze surface (via migration of soluble oxides). They emphasized that theirs was a consistent, reliable raw material with fewer impurities to throw colors off.
Their recommendations for using it were:
- Do not use excess water in mixing the glaze.
- Store the wet glaze in an airtight container.
- Always test first it in a small batch.
- Test on the same clay body you will use in production.
- Test on vertical tiles placed throughout the kiln.
As to the recipe, they hinted it was "comprised principally of ulexite (sodium calcium borate), with small amounts of colemanite (calcium borate) and approximately 10% colloidal clay-like materials". They claimed to add "various clay minerals" (vs Boraq that only used hectorite). In addition, they said the product contained "alkaline earth carbonates and silicates" (like talc and calcium carbonate). We got an exact analysis early, but they later said the product was "approximately 25% B2O3, 23% CaO, 4% total alkali and 31% loss on ignition". For some reason, they did not state the amount of SiO2, it would have to be at least 10%.
It should be noted that the melting properties of Gerstley Borate were a product of its being a mix of ulexite and colemanite. They melt at distinctly separate temperatures, this is an important factor in the material's contribution to variegation. It is uncertain how well Gillespie Borate duplicates this aspect. Indications are that it tends to create blisters in glazes that did not do so using Gerstley (eg. the 50:30:20 recipe).
Related Information
Gerstley Borate 50:30:20 glaze using Gillespie Borate instead

This picture has its own page with more detail, click here to see it.
This is the G2826A 50:30:20 GB:kaolin:silica base clear recipe. It is been used for decades as a base for all kinds of glazes. It starts melting early enough for use on low-temperature earthenware and is widely used in the raku process. Yet it is also common at middle temperatures (obviously care must be taken or it will run off ware onto kiln shelves when fired to cone 5-6). These tests were fired to cone 6 using the PLC6DS schedule.
The samples on the left use Gerstley Borate, on the right Gillespie Borate. The GBMF test tiles (lower left and right) reveal how much off-gassing is still happening on both when melting starts (they are full of bubbles). The GLFL test (centre) shows the melt flow of the two glazes, it is very similar (normal glazes do not run off the end of the runway like this). The two porcelain test tiles show it to fire crystal clear (there is some pooling since these were applied too thick). There is thus good reason to believe that Gillespie Borate will work well in this class of recipes.
How does Gillespie Borate compare with the original Floating Blue recipe?

This picture has its own page with more detail, click here to see it.
The original Floating Blue recipe, our code number G2826R, has been popular for 50 years. But also troublesome (because of a fragile mechanism, poor slurry properties and inconsistencies in Gerstley Borate and rutile). Gillespie Borate, it's 2023 apparent successor, appears to solve most of its issues. These specimens of the recipe were fired using the cone 6 C6DHSC schedule. We have "vintage" Gerstley Borate from the 1990s, that is what was used here.
Top left: Floating Blue using Gerstley Borate (GB) (top) and Gillespie Borate bottom on a buff burning body.
Top right: Same but on a red burning body.
Centre: Melt fluidity GLFL test of the two glazes (GB) on the left.
Bottom: The two recipes and their calculated chemistries.
Clearly, the Floating Blue itself is firing greener than usual. And the Gillespie Borate version is much bluer. You may be used to something in between these two. The green tones could likely be restored by a reduction in the cobalt and increase in the iron oxide. The best news is that at 1.47 specific gravity, Gillespie Borate produces a far better slurry, there is no gelling. And no sign of settling into a hard layer.
The chemistry comparison at the bottom highlights some concerns, the difference is not insignificant. B2O3, Al2O3 and SiO2 are all lower (this could be part of the reason for the differences in color also). For better or worse, the melt fluidity is the same: Very high. This is likely because the percentage of Ulexite is higher (that melts better than Colemanite).
Ferro Frit 3134 is NOT A SUBSTITUTE for Gerstley Borate

This picture has its own page with more detail, click here to see it.
This frit, or any of similar chemistry (e.g. Fusion F-12) IS NOT A 1:1 SUBSTITUTE for Gerstley Borate, their chemistries are too different. That being said, the frit sources lots of B2O3, that makes it a candidate to weave into recipes as the source of boron. To show the difference I have put 100 parts of each in side-by-side recipes in my Insight-live.com account, set the calculation type to non-unity formula and increased the frit until the B2O3 in the two match. Notice it takes 118g of the frit to source the same amount of B2O3 as 100 GB. Notice also that the frit sources almost triple the amount of Na2O per weight unit (that is a big deal because it means the recipe containing the Gerstley Borate to be substituted needs an Na2O-sourcing material that can be reduced to compensate). And the frit sources 3.5 times the SiO2 (other SiO2-sourcing materials in the recipe will need to be cut to compensate). And the Gerstley Borate has significant MgO while the frit has none (so an MgO-sourcing material like talc will be needed). Minor tweaks will also be needed to reduce other sources of CaO (since the frit has quite a bit more). The recipe will also need enough flexibility to do the final matching of Al2O3 and SiO2. The GBMF test confirms the difference at 1700F, these 10-gram balls melted down onto the tiles very differently.
Gerstley Borate vs Frit 3134 melt fluidity comparison

This picture has its own page with more detail, click here to see it.
Here the melt fluidity of Gerstley Borate (GB) is being compared to Ferro Frit 3134 (using a GLFL test). Clearly, these are two very different materials. GB is a clay, Frit 3134 is a man made powdered glass. Notice the GB shrinks to about half its original size by 1600F and then suddenly by 1650 it has exploded out of the starting gate and crossed the finish line! The frit, conversely, slowly softens through the entire 1350-1650 range and then starts down the runway between 1650 and 1700F. While it is clear that frit 3134 is not a direct substitute for the Gerstley Borate (GB) it's more gradual melting make it a better source as a source of B2O3 (boron).
Why does Gerstley Borate melt in two stages? Because it is two minerals.
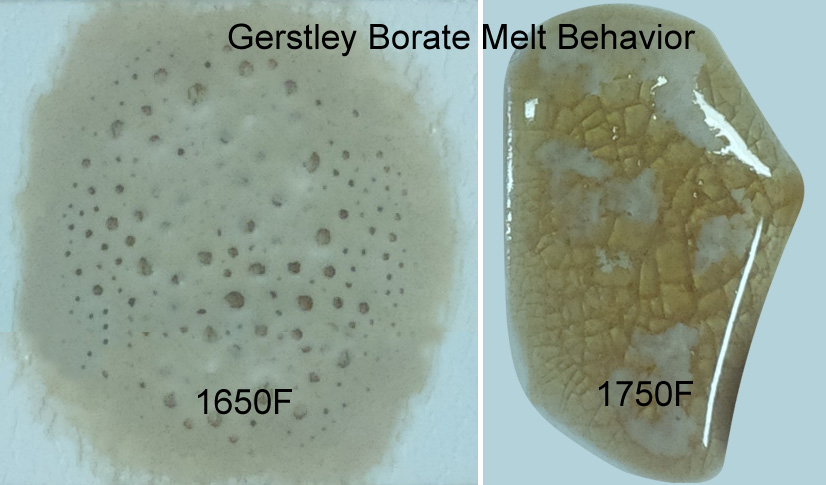
This picture has its own page with more detail, click here to see it.
The ulexite in Gerstley Borate melts first, producing an opaque fired glass having the unmelted (and still gassing) particles of colemanite suspended in it. By 1750F the colemanite is almost melted also. Boron-containing frits, by contrast, soften slowly over a wide temperature range and gradually spread and melt. Not surprisingly they produce a more stable glaze (albeit often less interesting visually without additives e.g. titanium, rutile). These behavior contributes to phase changes in fired glazes that contribute to variegation.
Here is why Gillespie Borate is crawling some glazes

This picture has its own page with more detail, click here to see it.
This is a variation on the 50:30:20 cone 6 pottery glaze recipe, it contains 22% Gillespie Borate (GB) and 12% calcined kaolin. Our objective was to reduce its melt fluidity. But the crawling is so bad that it is almost unusable. The reason was not obvious until we fired a sample to 1550F and 1650F. At the former the integrity of the glaze layer is great, but by 1650F it does this (many of the edges of these are curling upward). Ulexite, which GB contains, is known for the behaviour of suddenly shrinking and then suddenly melting over a narrow range of temperatures. Since GB is plastic and suspends slurries well we thought calcined kaolin would be better than raw kaolin (to minimize drying shrinkage). However, the improvement is minimal.
Gillespie Borate is doing something very strange at 1700F

This picture has its own page with more detail, click here to see it.
On the left is G2826A3, a cone 6 transparent glaze (an improvement on the 50:30:20 classic Gerstley Borate base transparent recipe, substituting Gillespie Borate, reducing its percentage and increasing SiO2). Despite the improvements it exhibits this strange cracking and crawling. The G2826A1 on the right uses a frit to source the boron instead, clearly a better idea. These tiles were fired to 1700F. The problem is likely the ulexite mineral in the Gillespie Borate - it is known for this behavior of suddenly shrinking and then suddenly melting (the latter of which is just starting). Since Gillespie Borate is plastic and suspends slurries well, I thought calcined kaolin would be better than raw kaolin in the G2826A3 recipe (to minimize drying shrinkage). However, it did not improve the situation. All of this being said, this recipe is still working reasonably well at cone 6 (stopping and holding it at 1700F may exaggerate the problem).
Gerstley Borate vs. Gillespie Borate at 1550F (840C)

This picture has its own page with more detail, click here to see it.
The GLFL test ball of pure Gerstley Borate has shrunk and vitrified to a porcelain state here at 1550F (the ball has shrunk to half the original size and gets even smaller by 1600F). Gerstley Borate has a significant LOI, it finishes off-gassing at about 1400F, which enables this high shrinkage that happens between 1350 and 1600F. Gillespie Borate, on the other hand, is obviously experiencing an overlap between the gassing and melting phases. What does that mean? It is already melting while gasses of decomposition are being expelled. Glazes having a high percentage of it are going to do this as they are heated through this range in a firing. It was not clear at first how this might affect glazes but it became evident later: Crawling.
Can you actually throw a Gerstley Borate glaze? Yes!

This picture has its own page with more detail, click here to see it.
G2931 Worthington Clear is a popular low to medium-fire transparent glaze recipe. It contains 55% Gerstley Borate (GB) plus 30% kaolin (GB melts at a very low temperature). GB is also very plastic, like a clay. I have thrown a pot from this glaze recipe! This explains why high Gerstley Borate glazes often dry so slowly and shrink and crack during drying. When recipes also contain a plastic clay like this one the shrinkage is even worse. GB is also slightly soluble, over time it gels glaze slurries even in smaller percentages. Countless potters struggle with Gerstley Borate recipes.
Links
| URLs |
http://www.hamgil.com
Hamil and Gillespie Website |
| URLs |
https://digitalfire.com/gb
GerstleyBorate.com - The best place for info on Gerstley Borate This site of the original gerstleyborate.com website, online from 2005-2025. This is the original content at a new address. |
| URLs |
https://digitalfire.com/4sight/datasheets/GillespieBorateFactSheet.pdf
Gillespie Borate fact sheet from Hammill and Gillespie. |
| Materials |
Boraq
This Gerstley Borate substitute was available during the early 2000s. Its recipe and development are well documented but two materials are no longer available. |
| Materials |
Laguna Borate
A Gerstley Borate substitute that was available during the early 2000s. |
| Materials |
Gerstley Borate
Gerstley Borate was a natural source of boron for ceramic glazes. It was plastic and melted clear at 1750F. Now we need to replace it. How? |
| Typecodes |
Gerstley Borate Substitutes
Many development efforts to create Gerstley Borate substitutes took place during the early 2000s (the initial period when the demise of Gerstley Borate appeared imminent). A number of companies, including Laguna Clays itself, produced and sold these for many years. When Laguna secured another stockpile at the mine and began producing the original material again, interest in substitutes gradually waned. However, the sudden dramatic price increase in 2023 appears to have initiated the process again. Gillespie Borate appears to be the only viable and visible substitute now. Thus, the substitutes listed here are mostly no longer made. Other high-boron materials shown are also no longer available. We continue to recommend sourcing B2O3 from frits instead. Please contact us if you have a specific recipe and we can work with you in your Insight-live account to develop a new recipe that both eliminates the GB and improves overall working and firing properties. |
| Media |
Getting Frustrated With a 55% Gerstley Borate Glaze
I show you why people love/hate this material and how I substituted it for Ulexite in this crazy recipe to make a far easier-to-use slurry that fires identical. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
