| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Colemanite
Description: Calcium borate mineral
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 27.30% | 1.00 | |
| B2O3 | 50.80% | 1.50 | |
| LOI | 21.90% | n/a | |
| Oxide Weight | 160.49 | ||
| Formula Weight | 205.49 | ||
Notes
Colemanite has been a popular natural source of insoluble boron for many decades. It is similar to Ulexite in its oxide contribution to glazes (although the latter sources Na2O also). Frits are used as boron sources in industry whereas potters and smaller companies have used colemanite.
Colemanite does not melt as low or as uniformly as Ulexite. Gerstley Borate contains significant amounts of colemanite. Pure colemanite, however, is much higher in B2O3 than Gerstley Borate.
Higher percentages of colemanite in a glaze can result in wrinkling of the fired surface, likely due to to a phenomenon called 'decrepitation' (very active decomposition) that occurs when colemanite is heated. The glaze layer can actually delayer, even disintegrate if sufficient colemanite is present (pieces of glaze can be spit off the ware onto other ware or the kiln shelf. For many applications Ulexite is thus a better choice. If you must use Colemanite, be sure to screen out any materials coarser than 200 mesh, or ball mill the glaze. Gum or other binders also help.
Colemanite is available from Turkey, Chile and California. The chemistry of these varies quite a bit, and of course, none of the available materials have the theoretical chemistry shown here.
Related Information
This high-colemanite underglaze has decrepitated, ruining the overglaze

This picture has its own page with more detail, click here to see it.
The Colemanite-based black underglaze over which a raspberry non-colemanite glaze was poured resulting in severe crawling as the Colemanite exfoliated and detached the overglaze. Courtesy of Nigel Hicken.
Colemanite and what its decrepitation does in glazes

This picture has its own page with more detail, click here to see it.
Decrepitation refers to a decomposition accompanied by scaling, delayering, even disintegration of the glaze layer. Moving rightward these glazes have increasing percentages of colemanite. At its worst (far right) the glaze is spattering off the sample and onto the kiln shelf. The others are crawling, first pulling away from the corners (far left) moving toward pulling away on the flat surfaces (center). Gerstley Borate and Ulexite, similar minerals, are far less likely to do this (but they have other serious issues also). A much better solution is to use frits to source the oxide B2O3 (easy to do in your account at Insight-live.com). Photos courtesy of Nigel Hicken.
Why does Gerstley Borate melt in two stages? Because it is two minerals.
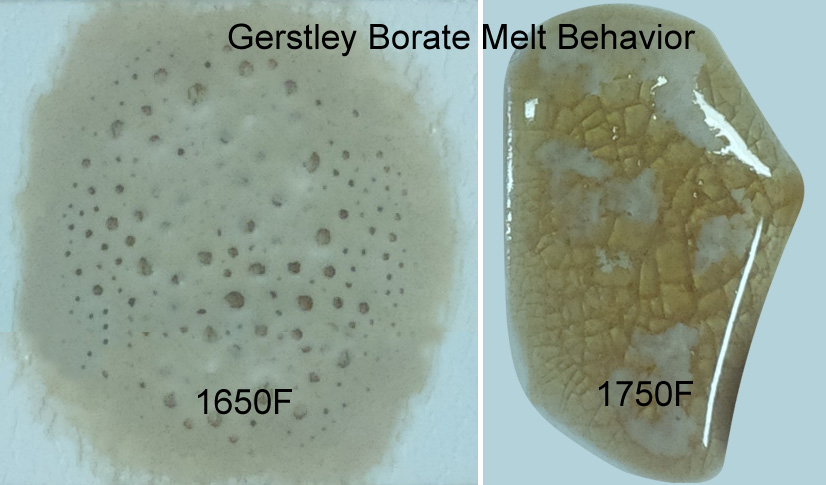
This picture has its own page with more detail, click here to see it.
The ulexite in Gerstley Borate melts first, producing an opaque fired glass having the unmelted (and still gassing) particles of colemanite suspended in it. By 1750F the colemanite is almost melted also. Boron-containing frits, by contrast, soften slowly over a wide temperature range and gradually spread and melt. Not surprisingly they produce a more stable glaze (albeit often less interesting visually without additives e.g. titanium, rutile). These behavior contributes to phase changes in fired glazes that contribute to variegation.
Links
| URLs |
https://digitalfire.com/gb
GerstleyBorate.com - The best place for info on Gerstley Borate This site of the original gerstleyborate.com website, online from 2005-2025. This is the original content at a new address. |
| URLs |
http://en.wikipedia.org/wiki/Colemanite
Colemanite at Wikipedia |
| Materials |
Gerstley Borate
Gerstley Borate was a natural source of boron for ceramic glazes. It was plastic and melted clear at 1750F. Now we need to replace it. How? |
| Materials |
Turkish Ulexite
A mineral with very high B2O3 and CaO content. |
| Materials |
Cadycal
|
| Materials |
Ulexite
A natural source of boron, it melts at a very low temperature to a clear glass. |
| Materials |
PotteryCrafts Frit P2954
A calcium borate frit (with extremely high boron). |
| Materials |
Ferro Frit 4112
A calcium borate frit (with extremely high boron). |
| Materials |
Turkish Colemanite
A mineral with very high B2O3 and CaO content. |
| Materials |
ABC Colemanite
|
| Materials |
Colemanite 38
|
| Materials |
Colemanite AM
|
| Materials |
Calcium Borate
|
| Typecodes |
Generic Material
Generic materials are those with no brand name. Normally they are theoretical, the chemistry portrays what a specimen would be if it had no contamination. Generic materials are helpful in educational situations where students need to study material theory (later they graduate to dealing with real world materials). They are also helpful where the chemistry of an actual material is not known. Often the accuracy of calculations is sufficient using generic materials. |
| Typecodes |
Flux Source
Materials that source Na2O, K2O, Li2O, CaO, MgO and other fluxes but are not feldspars or frits. Remember that materials can be flux sources but also perform many other roles. For example, talc is a flux in high temperature glazes, but a matting agent in low temperatures ones. It can also be a flux, a filler and an expansion increaser in bodies. |
| Typecodes |
Gerstley Borate Substitutes
Many development efforts to create Gerstley Borate substitutes took place during the early 2000s (the initial period when the demise of Gerstley Borate appeared imminent). A number of companies, including Laguna Clays itself, produced and sold these for many years. When Laguna secured another stockpile at the mine and began producing the original material again, interest in substitutes gradually waned. However, the sudden dramatic price increase in 2023 appears to have initiated the process again. Gillespie Borate appears to be the only viable and visible substitute now. Thus, the substitutes listed here are mostly no longer made. Other high-boron materials shown are also no longer available. We continue to recommend sourcing B2O3 from frits instead. Please contact us if you have a specific recipe and we can work with you in your Insight-live account to develop a new recipe that both eliminates the GB and improves overall working and firing properties. |
| Oxides | B2O3 - Boric Oxide |
| Oxides | CaO - Calcium Oxide, Calcia |
| Minerals |
Borate Minerals
The major borate minerals are Colemanite and Ulexite. The geology required for borates is found in v |
| Glossary |
Boron Frit
Most ceramic glazes contain B2O3 as the main melter. This oxide is supplied by great variety of frits, thousands of which are available around the world. |
Data
| Hardness (Moh) | 4.5 |
|---|---|
| Frit Softening Point | 1050C |
| Solubility | in HCl but not in water |
| Density (Specific Gravity) | 2.95 |
| Density (Specific Gravity) | 2.4 |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
