| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Ulexite
Alternate Names: Television Stone
Description: Sodium calcium borate mineral
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 13.84% | 0.67 | |
| Na2O | 7.65% | 0.33 | |
| B2O3 | 42.95% | 1.67 | |
| LOI | 35.55% | n/a | |
| Oxide Weight | 174.12 | ||
| Formula Weight | 270.16 | ||
Notes
Ulexite is a natural source of boron, it is similar to colemanite mineral. These two minerals are the only practical sources of insoluble boron for glazes (other than frits). Ulexite one of the lowest melting non-lead ceramic minerals, it can form an ultra-gloss transparent glass at cone 06. Strangely this material does not appear to flux bodies nearly as well as one might expect.
Ulexite is a truly uncommon ceramic mineral in that it contains almost no alumina or silica, it is nothing but fluxing oxides. This mineral forms in unusual geologic circumstances and can be found in very few places in the world. The chemistry given here is theoretical, actual deposits will have lower boron content.
The popular mineral Gerstley Borate, used by potters for many decades is, is composed partly of ulexite. It was mined and stockpiled in the California desert many years ago. The last remaining stockpile is being ground, bagged and sold by Laguna Clay.
While Ulexite melts well, it does have a very high LOI (that means that gases are generated during melting). For this reason, it is not possible to produce ultra-clear glazes at low temperatures (as with frits). Notwithstanding this, by doing a drop-and-hold firing it is possible to clear many more bubbles. Thinner glaze layering is also much more transparent.
Ulexite is available industrially from Turkey and Chile. It can be found in the wild in California. It is used in the fiberglass industry as a melter but its potential has never been exploited to any extent in ceramic glazes.
Related Information
Why does Gerstley Borate melt in two stages? Because it is two minerals.
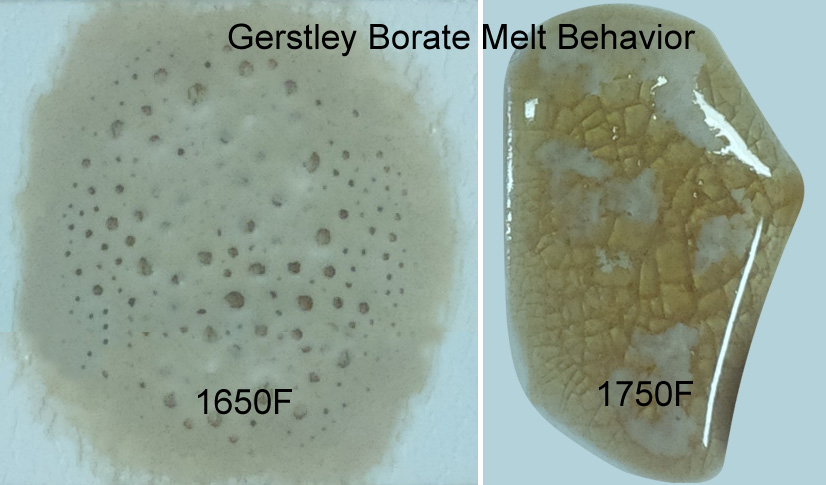
This picture has its own page with more detail, click here to see it.
The ulexite in Gerstley Borate melts first, producing an opaque fired glass having the unmelted (and still gassing) particles of colemanite suspended in it. By 1750F the colemanite is almost melted also. Boron-containing frits, by contrast, soften slowly over a wide temperature range and gradually spread and melt. Not surprisingly they produce a more stable glaze (albeit often less interesting visually without additives e.g. titanium, rutile). These behavior contributes to phase changes in fired glazes that contribute to variegation.
Ulexite rocks for sale on eBay

This picture has its own page with more detail, click here to see it.
There are many others selling them. It should be possible to buy rejects from them and grind them up to make your own Ulexite powder.
The difference between these low fire transparents: Gerstley Borate vs. Ulexite

This picture has its own page with more detail, click here to see it.
Left: Worthington Clear cone 04 glaze (A) uses Gerstley Borate to supply the B2O3 and CaO. Right: A substitute using Ulexite and 12% calcium carbonate (B). The degree of melting is the same but the gassing of the calcium carbonate has disrupted the flow of B. Gerstley Borate gasses also, but does so at a stage in the firing that does not disrupt this recipe. However, as a glaze, B does not gel and produces a clearer glass. A further adjustment to source CaO from non-gassing wollastonite would likely improve it.
A cure for long-time Gerstley Borate sufferers

This picture has its own page with more detail, click here to see it.
These are various different terra cotta clays fired to cone 04 with a recipe I developed that sources the same chemistry as the popular G2931 Worthington clear (50:30:20 GB:Kaolin:Silica) but from a different set of materials. The key change was that instead of getting the B2O3 from Gerstley Borate I sourced it first from Ulexite (G2931B) and then from a mix of frits (G2931K). All pieces were fired with a drop-and-hold firing schedule C03DRH. Fit was good on many terra cottas I tried (pieces even surviving boiling:icewater stressing). Where it did not fit I had thermal expansion adjustability because more than one frit was sourcing the boron. Frits are so much better for sourcing B2O3 than Gerstley Borate (the latter is notorious for turning glaze slurries into jelly!). Of course, a little glaze chemistry is needed to figure out how to convert a recipe from Gerstley Borate bondage to frit freedom, but there is lots of information here on how to do that.
Alberta slip and Ulexite at cone 6

This picture has its own page with more detail, click here to see it.
90% Alberta Slip (which is a mix of half and half raw and calcine) and 10% Ulexite fired at cone 6. A dazzling fluid dark amber transparent. You could also do this using a high-boron frit.
The perfect storm to create boron-blue clouding at low fire

This picture has its own page with more detail, click here to see it.
Two clear glazes fired in the same slow-cool kiln on the same body with the same thickness. Why is one suffering boron blue (1916Q) and the other is not? Chemistry and material sourcing. Boron blue crystals grow best when there is plenty of boron (and other power fluxes), alumina is low, adequate silica is available and cooling is slow enough to give them time to grow. In the glaze on the left B2O3 is higher, crystal-fighting Al2O3 and MgO levels are a lot lower, KNaO fluxing is significantly higher, it has more SiO2 and the cooling is slow. In addition, it is sourcing B2O3 from a frit making the boron even more available for crystal formation (the glaze on the right is G2931F, it sources its boron from Ulexite).
Two transparents having opposite melt fluidity/surface tension balances

This picture has its own page with more detail, click here to see it.
This cone 04 flow tester compares two commercial low-fire transparent glazes. Their different approaches to the chemistry are revealed by these melt flows. While 3825B appears to have a higher melt fluidity, its higher surface tension is the real story. This is demonstrated by how the flow meets the runway at a perpendicular angle. Notice that A, by contrast, meanders down the runway in a broad, flat and relatively bubble-free river. Low-fire glazes must pass many more bubbles than their high-temperature counterparts, the low surface tension of A aids in that. A is Amaco LG-10. B is Crysanthos SG213 (Spectrum 700 behaves similarly, although flowing less). Both have advantages and disadvantages and are worth testing in your application.
Links
| URLs |
http://mineral.galleries.com/minerals/carbonat/ulexite/ulexite.htm
The Mineral Ulexite |
| URLs |
http://en.wikipedia.org/wiki/Ulexite
Ulexite at Wikipedia |
| URLs |
http://www.americanborate.com/boron-products/ulexite/
Ulexite information at American Borates |
| URLs |
http://www.electronicsandbooks.com/eab1/manual/Magazine/T/Thermochimica%20Acta/336_400/S0040603100005840.pdf
Ulexite decomposition on heating |
| URLs |
https://borates.today/ulexite-the-wonder-mineral
Details on ulexite from Borates.today |
| Materials |
Gerstley Borate
Gerstley Borate was a natural source of boron for ceramic glazes. It was plastic and melted clear at 1750F. Now we need to replace it. How? |
| Materials |
Calcium Borate
|
| Materials |
ABC Colemanite
|
| Materials |
Colemanite
A natural source of boron that melts at a very low temperature. |
| Materials |
Turkish Colemanite
A mineral with very high B2O3 and CaO content. |
| Materials |
Chilean Ulexite
|
| Materials |
Turkish Ulexite
A mineral with very high B2O3 and CaO content. |
| Materials |
Hydroboracite 30
|
| Typecodes |
Generic Material
Generic materials are those with no brand name. Normally they are theoretical, the chemistry portrays what a specimen would be if it had no contamination. Generic materials are helpful in educational situations where students need to study material theory (later they graduate to dealing with real world materials). They are also helpful where the chemistry of an actual material is not known. Often the accuracy of calculations is sufficient using generic materials. |
| Typecodes |
Flux Source
Materials that source Na2O, K2O, Li2O, CaO, MgO and other fluxes but are not feldspars or frits. Remember that materials can be flux sources but also perform many other roles. For example, talc is a flux in high temperature glazes, but a matting agent in low temperatures ones. It can also be a flux, a filler and an expansion increaser in bodies. |
| Typecodes |
Gerstley Borate Substitutes
Many development efforts to create Gerstley Borate substitutes took place during the early 2000s (the initial period when the demise of Gerstley Borate appeared imminent). A number of companies, including Laguna Clays itself, produced and sold these for many years. When Laguna secured another stockpile at the mine and began producing the original material again, interest in substitutes gradually waned. However, the sudden dramatic price increase in 2023 appears to have initiated the process again. Gillespie Borate appears to be the only viable and visible substitute now. Thus, the substitutes listed here are mostly no longer made. Other high-boron materials shown are also no longer available. We continue to recommend sourcing B2O3 from frits instead. Please contact us if you have a specific recipe and we can work with you in your Insight-live account to develop a new recipe that both eliminates the GB and improves overall working and firing properties. |
| Glossary |
Borate
Borate glazes, those fluxed with the oxide B2O3, are the most common type used in ceramic industry and hobby for low and medium temperatures. |
| Glossary |
Boron Frit
Most ceramic glazes contain B2O3 as the main melter. This oxide is supplied by great variety of frits, thousands of which are available around the world. |
| Oxides | B2O3 - Boric Oxide |
| Minerals |
Borate Minerals
The major borate minerals are Colemanite and Ulexite. The geology required for borates is found in v |
| Media |
Getting Frustrated With a 55% Gerstley Borate Glaze
I show you why people love/hate this material and how I substituted it for Ulexite in this crazy recipe to make a far easier-to-use slurry that fires identical. |
| Firing Schedules |
Cone 6 Drop-and-Soak Firing Schedule
350F/hr to 2100F, 108/hr to 2200, hold 10 minutes, freefall to 2100, hold 30 minutes, free fall |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
