| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Ferro Frit 3110
Alternate Names: F3110, Frit KFG 4110, Ferro Crystal Frit, Ferro Frit 3110-2
Description: High alkali, low alumina and boron leadless high expansion frit
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 6.29% | 0.29 | |
| K2O | 2.36% | 0.07 | |
| Na2O | 15.24% | 0.64 | |
| B2O3 | 2.64% | 0.10 | |
| Al2O3 | 3.70% | 0.09 | |
| SiO2 | 69.77% | 3.03 | |
| Oxide Weight | 261.11 | ||
| Formula Weight | 261.11 | ||
Notes
This is a USA pottery frit, Ferro now calls it Frit 3110-2.
Soft sodium borosilicate frit for glazes. Since it is a good source of Na2O it is very commonly found in glaze recipes all temperatures. It has a high thermal expansion, therefore useful for substitution into glazes that are shivering (e.g. the G1916Q recipe trades it for frit 3195 to increase expansion). Often used in crystal glazes.
Since it is somewhat soluble some precipitation can occur in glazes stored for lengthy periods.
This frit can be very useful to reduce the feldspar content in glazes (since many high feldspar glazes have low clay content and therefore poor slurry suspension properties and dried hardness). The chemistry of this frit is similar to a feldspar (but with low alumina and CaO in addition to the alkali fluxes). That means if least part of the feldspar can be substituted for Frit 3110 you can increase the kaolin (to supply the alumina) and thereby improve slurry properties (we have done this with excellent success up to cone 10R). In addition, you will be able to reduce the amount of troublesome calcium carbonate. Of course, glaze chemistry is needed to calculate how to do this, there are videos at digitalfire.com on how to do this.
It can be used with 3403 for bright and semi-matte wall tile glazes.
This frit is also good for use as a body flux to substitute for feldspar, much lower vitrifying ranges are possible. However, it is somewhat soluble, so bodies should be used soon after making them. Adding only a small amount, e.g. 5%, to a terra cotta body not only greatly improves the maturity, but can potentially reduce crazing and make glazes easier to fit.
Cone Range: 08-8
Fusion temperature: 1400F
Flow temperature: 1700F
This frit has a very low melting point like 3124, 3134, 3185.
Formerly Frit 1078
Related Information
These common Ferro frits have distinct uses in traditional ceramics

This picture has its own page with more detail, click here to see it.
I used Veegum to form 10 gram GBMF test balls and fired them at cone 08 (1700F). Frits melt really well, they do have an LOI like raw materials. These contain boron (B2O3), it is a low expansion super-melter that raw materials don’t have. Frit 3124 (glossy) and 3195 (silky matte) are balanced-chemistry bases (just add 10-15% kaolin for a cone 04 glaze, or more silica+kaolin to go higher). Consider Frit 3110 a man-made low-Al2O3 super feldspar. Its high-sodium makes it high thermal expansion. It works really well in bodies and is great to make glazes that craze. The high-MgO Frit 3249 (made for the abrasives industry) has a very-low expansion, it is great for fixing crazing glazes. Frit 3134 is similar to 3124 but without Al2O3. Use it where the glaze does not need more Al2O3 (e.g. already has enough clay). It is no accident that these are used by potters in North America, they complement each other well (equivalents are made around the world by others). The Gerstley Borate is a natural source of boron (with issues frits do not have).
Frits melt so much better than raw materials

This picture has its own page with more detail, click here to see it.
Feldspar and talc are both flux sources (glaze melters), they are common in all types of stoneware glazes. But their fluxing oxides, Na2O and MgO, are locked in crystal structures that neither melt early or supply other oxides with which they like to interact. The pure feldspar is only beginning to soften at cone 6. Yet the soda frit is already very active at cone 06! As high as cone 6, talc (the best source of MgO) shows no signs of melting activity at all. But a high-MgO frit is melting beautifully at cone 06! The frits progressively soften, starting from low temperatures, both because they have been premelted and have significant boron content. In both, the Na2O and MgO are free to impose themselves as fluxes, actively participating in the softening process.
Five frits. One kiln at 1850F. Big chemistry drama.

This picture has its own page with more detail, click here to see it.
Five common North American Ferro Frits fired at 1850F on alumina tiles (each started as a 10-gram GBMF test ball and flattened during the firing). At this temperature, the differences are more evident than at 1950F. The degree of melting corresponds mainly, but not only, to the percentage of B2O3 present. Frit 3134 is the runaway leader because it couples that with almost no Al2O3 to stabilize the melt. Notice also the crack pattern, it is also very high in Na2O and thus has a high COE. However, why does Frit 3110 melt so well even though it contains almost no B2O3? Even higher Na2O, it is a powerful flux. Its COE is even higher than Frit 3134, but it is thick enough to have resisted cracking far (but it will in time).
Melting glaze balls at various temperatures to see when all carbon has been expelled
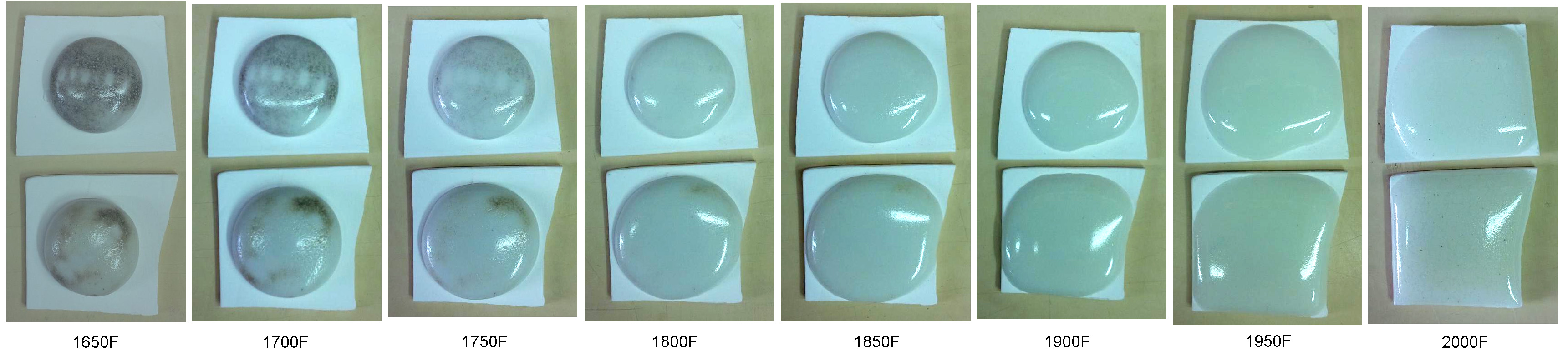
This picture has its own page with more detail, click here to see it.
G1916Q and J low fire ultra-clear glazes (contain Ferro Frit 3195, 3110 and clay) fired across the range of 1650 to 2000F (these were 10 gram GBMF test balls that melted and flattened as they fired). Notice how they soften over a wide range, starting below cone 010 (1700F)! At the early stages carbon material is still visible (even though the glaze has lost 2% of its weight to this point), it is likely the source of the micro-bubbles that completely opacify the matrix even at 1950F (cone 04). This is an 85% fritted glaze, yet it still has carbon - think of what a raw glaze might have! Of course, these specimens test a very thick layer, so the bubbles are expected. But they still can be an issue, even in a thin glaze layer on a piece of ware. So to get the most transparent possible result it is wise to fire tests to find the point where the glaze starts to soften (in this case 1450F), then soak the kiln just below that (on the way up) to fire away as much of the carbon as possible. Of course, the glaze must have a low enough surface tension to release the bubbles, that is a separate issue.
Frit melt fluidity comparison - 1300F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1300F and held for 15 minutes. Some are still burning off carbon (which seems strange). There are two early leaders: Ferro frit 3110 and Fusion frit F75 are starting to deform (they have almost the same chemistry). Amazingly, these two frits have low boron, they rely on high soda as the flux.
Melt fluidity comparison - 1750F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1750F and held for 15 minutes. Frit 3110 has taken off. And F75, 3195 and 3134 (the latter two having big differences in surface tension).
Melt fluidity comparison of frits - 1350F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1350F and held for 15 minutes. Some are still burning off carbon (which seems strange). The two FZ16s are starting to move. Frit 3134 is expanding. 3602 is also starting to melt.
Melt fluidity comparison of frits - 1400F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1400F and held for 15 minutes. Frit 3134 is still expanding. 3602 is also starting to flow. A number of them are shrinking and densifying like a porcelain would.
A Redart cone 03 body shines when it come to ease of glaze fit

This picture has its own page with more detail, click here to see it.
These bowls are fired at cone 03. They are made from 80 Redart, 20 Ball clay. The glazes are (left to right) G1916J (Frit 3195 85, EPK 15), G191Q (Frit 3195 65, Frit 3110 20, EPK 15) and G1916T (Frit 3195 65, Frit 3249 20, EPK 15). The latter is the most transparent and brilliant, even though that frit has high MgO. The center one has a higher expansion (because of the Frit 3110) and the right one a lower expansion (because of the Frit 3249). Yet all of them survived a 300F to icewater IWCT test without crazing. This is a testament to the utility of Redart at low temperatures. A white body done at the same time crazed the left two.
A high feldspar glaze is settling, running and crazing. What to do?

This picture has its own page with more detail, click here to see it.
The original cone 6 recipe, WCB, fires to a beautiful brilliant deep blue green (shown in column 2 of this Insight-live screen-shot). But it is crazing and settling badly in the bucket. The crazing is because of high KNaO (potassium and sodium from the high feldspar). The settling is because there is almost no clay in the recipe. Adjustment 1 (column 3 in the picture) eliminates the feldspar and sources Al2O3 from kaolin and KNaO from Frit 3110 (preserving the glaze's chemistry). To make that happen the amounts of other materials had to be juggled. But the fired test revealed that this one, although very similar, is melting more (because the frit releases its oxides more readily than feldspar). Adjustment 2 (column 4) proposes a 10-part silica addition. SiO2 is the glass former, the more a glaze will accept without losing the intended visual character, the better. The result is less running and more durability and resistance to leaching.
1700F Frit Melt-Off: Who is the winner? Not the lead bisilicate!

This picture has its own page with more detail, click here to see it.
These were 10g balls melted using our GBMF test. We fired at a temperature far lower than typical bisque, notice how many of them are already melting well! Frit 3602 is lead bisilicate. But it got "smoked" by the Fusion FZ-16 high-zinc, high-boron zero-alumina! Maybe you always thought lead was the best melter. That it produced the most transparent, crystal-clear glass. But that is not what we see here. That being said, notice the lead is not crazing but the FZ-16 is crazing badly, that is a problem for many applications using this frit, it relies on a high percentage of KNaO. Notice something else: Each frit has a distinctive melt fingerprint that makes it recognizable in tests like this. Want to get some of this frit for pottery? You can't, Fusion Ceramics doesn't want to handle retail sales of smaller quantities.
The first of 15 "Fool-Proof Recipes" wrecked my kiln shelf!

This picture has its own page with more detail, click here to see it.
This is recommended in the booklet "15 Tried and True Cone 6 Glaze Recipes". This melt flow tester compares it with a typical cone 6 glossy, G2926B. This recipe is 90% Frit 3110 and 10% kaolin and their booklet recommends adding stains to it. But anyone knowing a little about this frit knows it would run off this flow tester even before bisque temperatures. It is crazy to recommend this. Even as a crackle. For cone 6 it needs to be diluted much more, not just with kaolin but also silica. I knew this would run but I underestimated its melt fluidity. I put a large tile below the tester to catch overrun, yet the melt ran off that and a big blob melted through the kiln wash and so far into the zircon shelf I could not chip it off! I cannot imagine how many people have tried this on vertical surfaces and had the same thing happen. The lesson: Use common sense when looking at recipes, then you don't even need to waste time testing them. Even if their authors did not!
Melt fluidity comparison of frits - 1450F
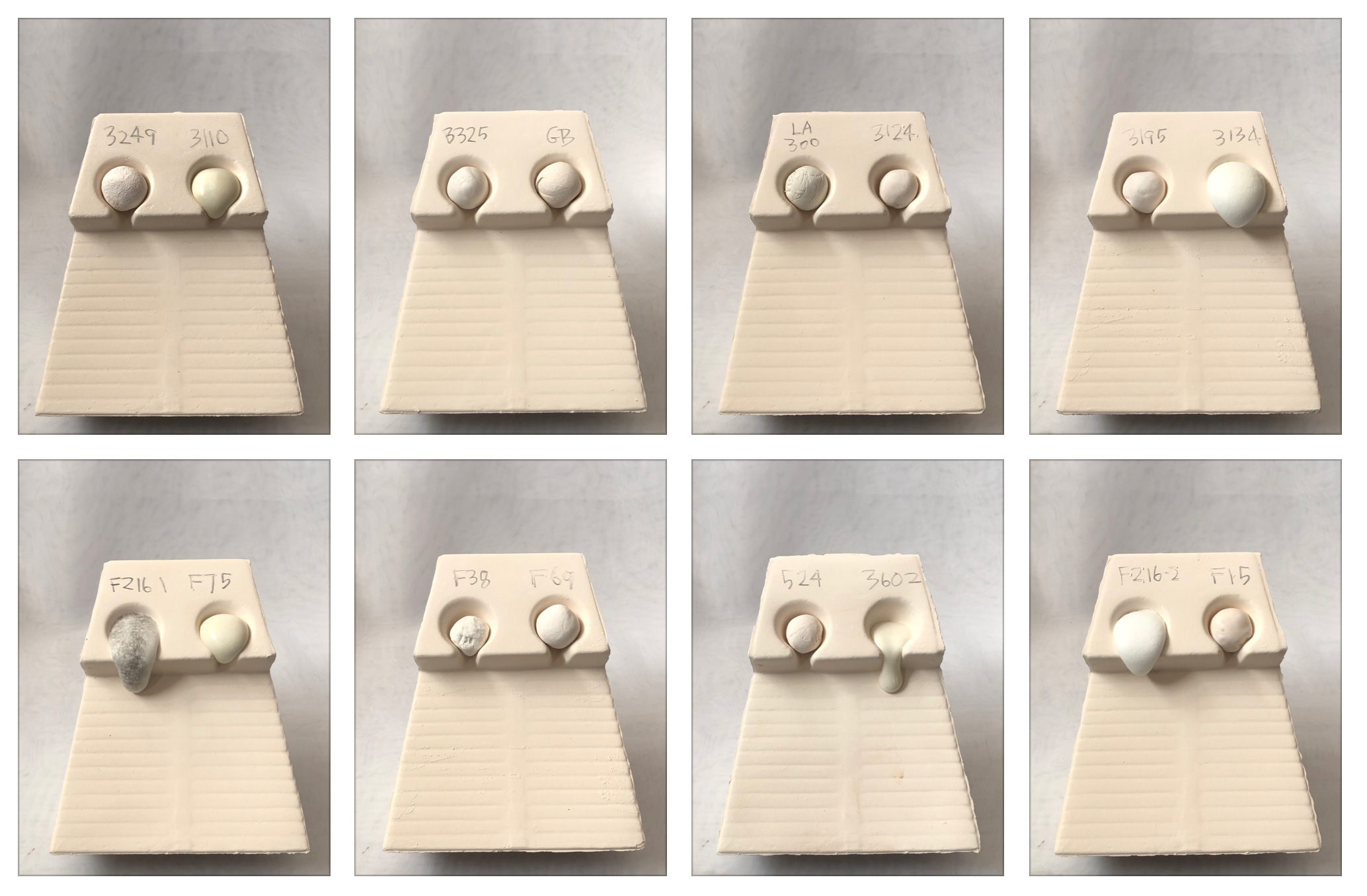
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1450F and held for 15 minutes. Frit 3134 is still expanding. 3602 is blasting out of the gate, taking the lead. F75 is starting to flow.
Melt fluidity comparison of frits - 1500F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1500F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are really starting to move. 3195, F38 and F15 are softening.
Melt fluidity comparison of frits - 1550F
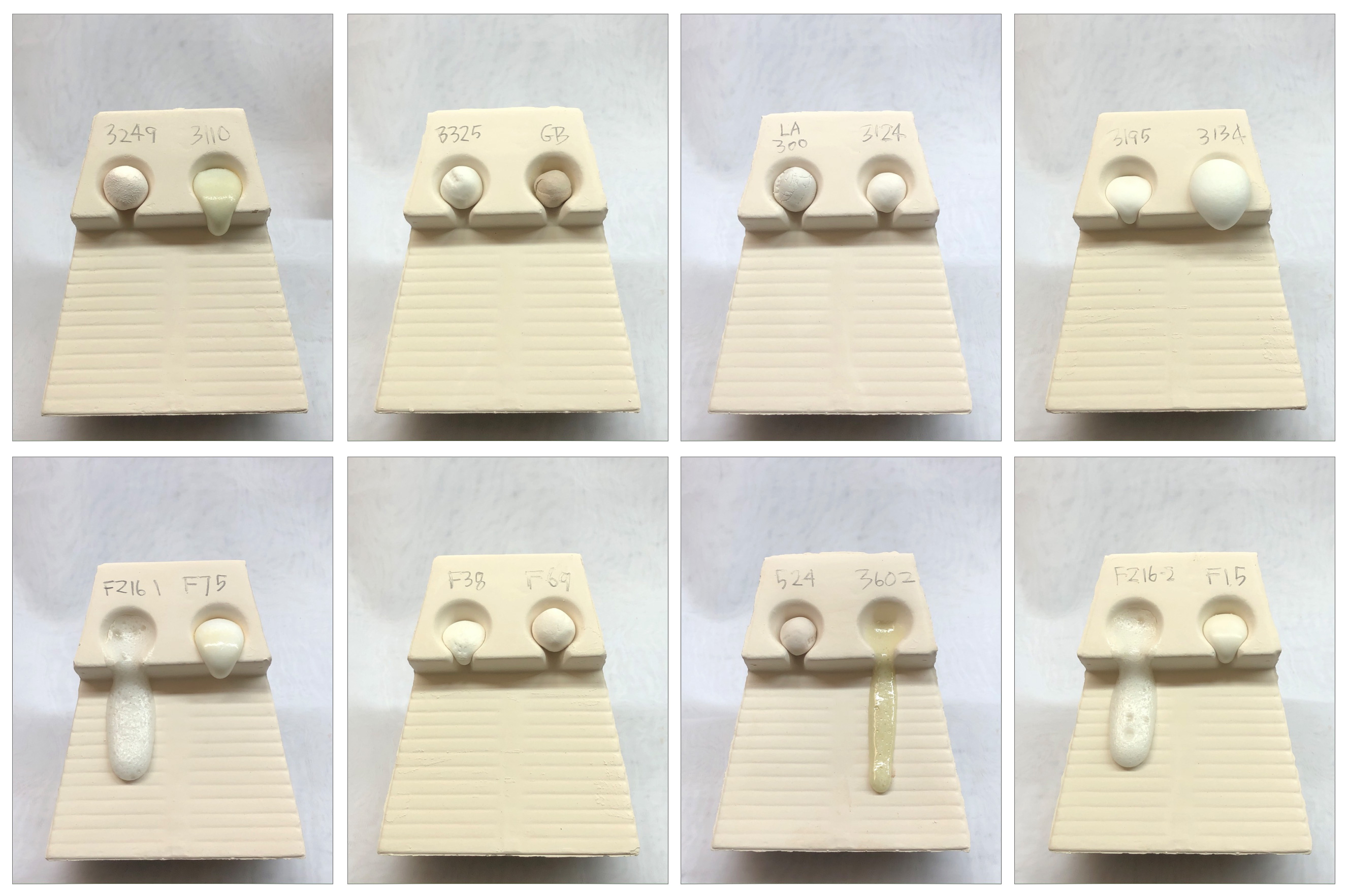
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1550F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are going to be off-ramp by next firing.
Melt fluidity comparison of frits - 1650F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1650F and held for 15 minutes. FZ16 has turned crystal clear and spread out across the runway (has low surface tension). Frit 3110 has so much surface tension that the flow can be lifted off the tester. Since 1600F Gerstley Borate has gone from unmelted to passing all the rest!
Melt fluidity comparison of frits - 1700F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1700F and held for 15 minutes. 3110 is finally starting to move. 3134 also (being full of bubbles). Gerstley Borate has turned almost transparent (because the Colemanite portion of it is now melting). 3195 is looking very well behaved compared to most others, forming a bubble free glass of high surface tension (F15 and F524 are starting to do the same).
Frit Melt Fluidity Comparison - 1800F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1800F and held for 15 minutes (I already did firings from 1300F-1750F in 50 degree increments, all of them are visible in the parent project). Frit 3110, 3134, 3195, F75 have run all the way down. All of the frits have softened and melted slowly over a range of temperatures (hundreds of degrees). By contrast, Gerstley Borate, the only raw material here, suddenly melted and flowed right over the cliff (between 1600 and1650)! But not before Frit 3602 and FZ16 had done so earlier. Frit 3249 is just starting to soften but F69 (the Fusion Frits equivalent) is a little ahead of it. LA300 and Frit 3124 are starting also. F524, F38, F15 will all be over the end by the next firing. The melt surface tension is evident by the way in which the melts spread out or hold together.
Frits vs. raw materials in glazes: It is not just about the chemistry

This picture has its own page with more detail, click here to see it.
The difference between sourcing fluxing oxides from frits vs. raw materials is significant to say the least. The oxides MgO and CaO normally come from natural mineral that melt high. But common frits that source them soften low. The chemistry in the two cone 6 glazes (compared on this melt flow tester) are the same. But G2934Y4 sources the KNaO from Ferro Frit 3110 instead of feldspar and alot of the MgO from Ferro Frit 3249 instead of talc. Even though Y4 has 10% calcined alumina it still flows much better! Alumina, as a source of Al2O3, is a super refractory material (compared to kaolin, the normal source), the glaze should flow less - but the frits overcome even that to create this amazing fluidity in a matte glaze. The lesson: All glazes have a chemistry, but that cannot be taken in isolation from the materials that source it. Glaze chemistry is a relative, not absolute science.
Ferro Frit 3110 vs Fusion F-75 at cone 04

This picture has its own page with more detail, click here to see it.
On paper, Fusion frit F-75 has a very similar chemistry to Ferro frit 3110. However, as can be seen here in this melt fluidity test, it is flowing a little more and appears to have a lower surface tension. The bubbling character is also a little different. The differences could be partly to Fusion using a different set of raw materials to source the chemistry. Or differences in their smelting process. This frit is commonly used in pottery to create crackle glazes or to help increase the thermal expansion of shivering glaze (both of these are cracking and crazing badly). Frit 3110 is also a major ingredient in crystalline glazes, the better clarity of the F-75 is going to help with that also. It is also used as a concentrated source of Na2O (instead of feldspar), also a task that this will certainly fulfill.
Frit Melt Fluidity Comparison - 1850F

This picture has its own page with more detail, click here to see it.
These melt flow tests were fired at 350F/hr to 1850F and held for 15 minutes (I did firings at 50-degree increments across a wide range). It is amazing how active some frits are, even well below normal bisque temperatures! Frit 3110, Frit 3134, Frit 3195, Frit F-75 have all flowed all the way down for many previous temperatures. LA300 and Frit 3124 were just starting at 1800F, look at them now! Frit F-524 and Frit F-38 have gone from half-way at 1800F to water-falling over the end. Frit 3249 is still not out-of-the-gate but Frit F-69 (the Fusion Frits equivalent of 3249) is half-way. Note how the melt surface tension is evident by the way in which the melts spread out or hold together. By contrast, Gerstley Borate (labelled "GB"), the only raw material here, suddenly melted and flowed right over-the-cliff between 1600 and 1650! The best melter of all of them is high-boron high-zinc Frit FZ-16.
Two frits with Custer Feldspar

This picture has its own page with more detail, click here to see it.
The GBMF test compares Custer Feldspar with 15% Frit 3110 and 15% Ferro Frit 3134. It is fired at cone 6.
Links
| Materials |
General Frit GF-134
|
| Materials |
Fusion Frit F-75
High sodium, high thermal expansion low boron frit. An equivalent of Ferro Frit 3110. |
| Materials |
Pemco Frit P-1V04
|
| Materials |
Frit
Frits are made by melting mixes of raw materials, quenching the melt in water, grinding the pebbles into a powder. Frits have chemistries raw materials cannot. |
| Materials |
Hommel Frit 529
|
| Materials |
Potclays Frit 2266
|
| Materials |
Ferro Frit 4110
|
| Materials |
PotteryCrafts Frit P3110
|
| Materials |
Frit 1078
|
| Materials |
Feldspar
In ceramics, feldspars are used in glazes and clay bodies. They vitrify stonewares and porcelains. They supply KNaO flux to glazes to help them melt. |
| Materials |
Ceradel Frit 3110
|
| Materials |
Fusion Frit F644
|
| Materials |
Keramikos Fritte 31.10
|
| Materials |
Potclays Frit 2275
|
| Materials |
Degussa Frit 90208
|
| Typecodes |
Frit
A frit is the powdered form a man-made glass. Frits are premelted, then ground to a glass. They have tightly controlled chemistries, they are available for glazes of all types. |
| Glossary |
Feldspar Glazes
Feldspar is a natural mineral that, by itself, is the most similar to a high temperature stoneware glaze. Thus it is common to see alot of it in glaze recipes. Actually, too much. |
| Glossary |
Crackle glaze
Crackle glazes have a crack pattern that is a product of thermal expansion mismatch between body and glaze. They are not suitable on functional ware. |
| Glossary |
Fritware
|
| URLs |
https://digitalfire.com/4sight/datasheets/ferropotteryfrits2008.pdf
Ferro Pottery Frits 2008 |
| Articles |
Formulating a Porcelain
The principles behind formulating a porcelain are quite simple. You just need to know the purpose of each material, a starting recipe and a testing regimen. |
Data
| Co-efficient of Linear Expansion | 10.08 |
|---|---|
| Frit Melting Range (C) | 1400-1700F |
Mechanisms
| Body Maturity | This frit is an excellent body flux, 15% can move a cone 10 body down to cone 6. |
|---|
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
