| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
A Low Cost Tester of Glaze Melt Fluidity
A One-speed Lab or Studio Slurry Mixer
A Textbook Cone 6 Matte Glaze With Problems
Adjusting Glaze Expansion by Calculation to Solve Shivering
Alberta Slip, 20 Years of Substitution for Albany Slip
An Overview of Ceramic Stains
Are You in Control of Your Production Process?
Are Your Glazes Food Safe or are They Leachable?
Attack on Glass: Corrosion Attack Mechanisms
Ball Milling Glazes, Bodies, Engobes
Binders for Ceramic Bodies
Bringing Out the Big Guns in Craze Control: MgO (G1215U)
Can We Help You Fix a Specific Problem?
Ceramic Glazes Today
Ceramic Material Nomenclature
Ceramic Tile Clay Body Formulation
Changing Our View of Glazes
Chemistry vs. Matrix Blending to Create Glazes from Native Materials
Concentrate on One Good Glaze
Copper Red Glazes
Crazing and Bacteria: Is There a Hazard?
Crazing in Stoneware Glazes: Treating the Causes, Not the Symptoms
Creating a Non-Glaze Ceramic Slip or Engobe
Creating Your Own Budget Glaze
Crystal Glazes: Understanding the Process and Materials
Deflocculants: A Detailed Overview
Demonstrating Glaze Fit Issues to Students
Diagnosing a Casting Problem at a Sanitaryware Plant
Drying Ceramics Without Cracks
Duplicating Albany Slip
Duplicating AP Green Fireclay
Electric Hobby Kilns: What You Need to Know
Fighting the Glaze Dragon
Firing Clay Test Bars
Firing: What Happens to Ceramic Ware in a Firing Kiln
First You See It Then You Don't: Raku Glaze Stability
Fixing a glaze that does not stay in suspension
Formulating a body using clays native to your area
Formulating a Clear Glaze Compatible with Chrome-Tin Stains
Formulating a Porcelain
Formulating Ash and Native-Material Glazes
G1214M Cone 5-7 20x5 glossy transparent glaze
G1214W Cone 6 transparent glaze
G1214Z Cone 6 matte glaze
G1916M Cone 06-04 transparent glaze
Getting the Glaze Color You Want: Working With Stains
Glaze and Body Pigments and Stains in the Ceramic Tile Industry
Glaze Chemistry Basics - Formula, Analysis, Mole%, Unity
Glaze chemistry using a frit of approximate analysis
Glaze Recipes: Formulate and Make Your Own Instead
Glaze Types, Formulation and Application in the Tile Industry
Having Your Glaze Tested for Toxic Metal Release
High Gloss Glazes
Hire Us for a 3D Printing Project
How a Material Chemical Analysis is Done
How desktop INSIGHT Deals With Unity, LOI and Formula Weight
How to Find and Test Your Own Native Clays
I have always done it this way!
Inkjet Decoration of Ceramic Tiles
Is Your Fired Ware Safe?
Leaching Cone 6 Glaze Case Study
Limit Formulas and Target Formulas
Low Budget Testing of Ceramic Glazes
Make Your Own Ball Mill Stand
Making Glaze Testing Cones
Monoporosa or Single Fired Wall Tiles
Organic Matter in Clays: Detailed Overview
Outdoor Weather Resistant Ceramics
Painting Glazes Rather Than Dipping or Spraying
Particle Size Distribution of Ceramic Powders
Porcelain Tile, Vitrified Tile
Rationalizing Conflicting Opinions About Plasticity
Ravenscrag Slip is Born
Recylcing Scrap Clay
Reducing the Firing Temperature of a Glaze From Cone 10 to 6
Setting up a Clay Testing Program in Your Company or Studio
Simple Physical Testing of Clays
Single Fire Glazing
Soluble Salts in Minerals: Detailed Overview
Some Keys to Dealing With Firing Cracks
Stoneware Casting Body Recipes
Substituting Cornwall Stone
Super-Refined Terra Sigillata
The Chemistry, Physics and Manufacturing of Glaze Frits
The Effect of Glaze Fit on Fired Ware Strength
The Four Levels on Which to View Ceramic Glazes
The Majolica Earthenware Process
The Potter's Prayer
The Right Chemistry for a Cone 6 Magnesia Matte
The Trials of Being the Only Technical Person in the Club
The Whining Stops Here: A Realistic Look at Clay Bodies
Those Unlabelled Bags and Buckets
Tiles and Mosaics for Potters
Toxicity of Firebricks Used in Ovens
Trafficking in Glaze Recipes
Understanding Ceramic Materials
Understanding Ceramic Oxides
Understanding Glaze Slurry Properties
Understanding the Deflocculation Process in Slip Casting
Understanding the Terra Cotta Slip Casting Recipes In North America
Understanding Thermal Expansion in Ceramic Glazes
Unwanted Crystallization in a Cone 6 Glaze
Using Dextrin, Glycerine and CMC Gum together
Volcanic Ash
What Determines a Glaze's Firing Temperature?
What is a Mole, Checking Out the Mole
What is the Glaze Dragon?
Where do I start in understanding glazes?
Why Textbook Glazes Are So Difficult
Working with children
The Effect of Glaze Fit on Fired Ware Strength
Description
The fit between body and glaze is like a marriage, if is is strong the marriage can survive problems. Likewise ceramic ware with well fitting glaze is much stronger than you think it might be, and vice versa.
Article
Shotgun marriages are not likely to last. Although this is true with people, I'm referring again to marriages between the glaze and clay on a fired piece of pottery. These two have to live together "for better and for worse". Most of us have always felt that "if they look good together" then everything is fine! However, continuing to ignore incompatibility won't make it go away. There will be a day of reckoning. Yes, I'm going to flog the subject of glaze fit one more time.
I have done tests that indicate it is possible for a good "marriage" of clay and glaze to double the strength of your unglazed ware. Conversely, a poor fitting glaze can cut it to one third of unglazed strength. That's one sixth of optimum! Incredibly, this range can occur without any visible difference in an otherwise attractive glaze, not even crazing. Are you using an attractive matte glaze like I was? Sometimes our ignorance can be such bliss when things look good! Many potters and technicians just do not want to know if there is a serious problem with their glaze. I am assuming you, however, would like to optimize things.
If the strength of the clay-glaze 'marriage' determines the strength of the entire piece, what can one do to get it off to a fine start, help them "become one" and stick together amidst trials and tribulations? Awareness is a good place to begin. Let's form a model to help us understand things better.
When clay objects are heated they expand; when cooled they contract. Since clay and glaze have to live attached to each other, they must expand and contract together. The degree of match between the two is referred to as "glaze-fit". A poor fitting glaze expands at a different rate than the clay to which it is attached. Usually there is no visible indication that there is a glaze fit problem, especially if the two are joined or interfaced well. But beneath that beautiful exterior, tension may threaten continued survival of the piece.
A glaze which is under some compression actually strengthens the ware. This is analogous to prestressed concrete where tension is maintained on steel reinforcing bars while concrete is poured and allowed to set. In my tests, I have noted an incredible fourfold variation in ware strength for a range of glaze expansions insufficient to cause even visible crazing or shivering. Thus, a visual examination is not enough to determine if a glaze is weakening or strengthening your ware, now or in the future.
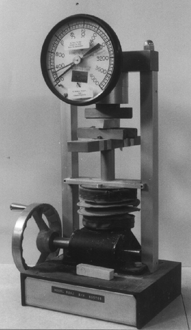
Round or square fired bars are subject to a force that tests their tensile strength. The strength can be calculated from the force and the dimensions of the cross section where the break occurred.
For many of us, the quality of the union between our clay and glazes is almost accidental. If someone claimed they could double the strength of your ware by changing the glaze recipe slightly, you would likely be all ears (yet it seems that it is possible to quadruple it in worst case situations!). If you have problems with easily chipped or broken ware, delayed crazing, ware losing the "ring" it had after firing, or ovenware cracking during use, then here is a methodology that can help. It is time to end the "buck passing", blaming the clay, and stop at the real source of the problem, the potter or glaze technician, the one holding the shot gun in this wedding!
One cannot improve glaze fit, and therefore ware strength, unless he can test it. Industry uses complex and expensive equipment to measure the expansion of fired glaze and clay samples over a range of temperatures. Orton, for example, manufactures dilatometers for this purpose. However, this is not really an ideal option for most. Not only is the expense prohibitive, but potters and small companies lack the expertise to use the methods and information to their benefit; to adapt it to their own unique circumstances. There are other less technical methods (e.g. fired rings, suspended strips) but if you've tried any of them, like me, you are less than impressed with the practical benefits.
I have a better way. Since the main purpose of refining a clay-glaze union is to assure continued harmony and therefore, maintenance of maximum ware strength, why not test its integrity by measuring their fired strength? Really, it seems to me that such a test is the very best, most applicable, and meaningful measure of clay/glaze compatibility. Rather than telling us what should happen when they are used together, it tells us what does happen.
Strength testing machines are normally simple hydraulic devices. They are used by many industries producing structural products. Different types of tests are done with them, including those for elasticity, compressive, and tensile strength. The Modulus of Rupture (M.O.R.) is of special interest. It is based on the amount of force necessary to break a bar at its mid point.
Based on the principles outlined below, it is possible to make a very simple strength testing device from a metal frame with a pivoting load that presses on the center of the bar that straddles two supports at each end. The loading arm has an attachment for a standard torque wrench with an inert needle. Once the device is set up you simply insert the specimen to be broken, press down on the wrench to break it, and record the force necessary to do so. This figure goes into an equation to compensate for the specifics of your device, then into the equation outlined below to produce the strength figure.
Modulus of Rupture
According to the ASTM (American Society for Testing and Materials) Handbook, test C 674- 77: "Specimens, either cylindrical, or rectangular, are supported on knife edges over a suitable span and a direct load is applied at the midpoint between the supports at a uniform rate until breakage occurs".
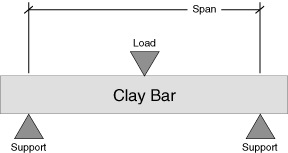
MOR - Modulus of Rupture
M=3PL/2bd2
Where:
- M=Modulus of Rupture in PSI,
P=Load at rupture in pounds,
L=Distance between supports (2 inches),
b=Width of specimen in inches,
d=Thickness of specimen in inches.
As already mentioned, there are two ways to carry out this test: 'By the book' on a commercial strength testing device or on a homemade 'gizmo' you can afford.
The best test bars are round and extruded directly through a die of the correct cross sectional size. As a second choice, the test bars can be cut lengthwise from an undisturbed slug of clay. The slug should be pugged in a good pugmill, having a properly designed compressing die, and preferably a de-airing chamber. Other methods of making them can introduce laminations and differences that will throw the strength results out. The bars should be dried slowly to prevent drying stresses and then bisque fired. If you are using a home made device, it is obvious there will be compromises. Specimens will have to be smaller and more easily broken, and you will have to tolerate some variation until you can find a way to make specimens that break with consistent force. One real advantage of a strength tester of this type is that you can also use it to evaluate the dry strength of clay bodies to rate their plasticity.
Now, let's get to the tests I have been talking about. Another potter, Clara Matthews, and I, wanted to find ways of making ovenware resistant to breakdown from thermal cycling, so we worked together to test the above theories. We selected two glazes, a typical fatty matte that potters drool over and a clear glossy. We will consider the matte here; it is by far the most likely to weaken your pots. Two clay bodies, a porcelain and a buff stoneware, were chosen and 8 bars of each clay were dipped into the glaze. These 16, plus 8 unglazed bars, were fired to cone 10R. Finally, they were broken in a commercial strength testing device and the M.O.R. of each group of 8 was calculated and averaged. Results of this initial breakage showed the buff stoneware (H550) at 3930 psi with glaze and 3950 without; the porcelain (P580) at 3810 with the glaze and 9122 without! These figures turn on some red lights! Watch out for glazes of this type on porcelain. A forced clay-glaze 'marriage' here, without meaningful compatibility checking, will likely be a bad one. It also seems that a porous stoneware, having the right glaze, can be as strong as a porcelain with a poor glaze. There are more surprises to come! Small changes in a glaze can dramatically improve ware strength.
The next step involves dreaded calculation! There is really no other viable way. One warning: Calculations can easily be misused and misapplied. The computer makes them so easy that sometimes we fail to maintain proper balance between the direction indicated by calculated results, and that pointed to by physical observations. For example, many use a calculation approach that seeks to harmonize calculated thermal expansions for clay and glaze.
This method is invalid because fired expansion characteristics developed by oxide combinations are as much dependent on firing methods, particle size, glass development, etc., as they are on the formula. The former are not taken into account in calculations. Further, the calculated expansion of a body or glaze will never equal its measured expansion. Don't get me wrong, the key to solving this problem lies with calculation but not with this particular calculation approach.
The real challenge here is to alter the expansion of the matte glaze by changing its recipe but, if possible, doing so without altering fired appearance. Basically, you start by substituting fluxes of higher or lower expansion while maintaining the overall balance of the glaze. You can take more drastic measures, such as adjusting the SiO2 , B2O3 , or Al2O3 where appropriate.
I started with INSIGHT by entering the original recipe and producing a detail printed report of its formula, showing the expansion contribution of each oxide and the composite calculated expansion.
Calculating Initial Expansion
DETAIL PRINT - Matte White Glaze MATERIAL PARTS WEIGHT CaO* MgO* K2O* Na2O* Fe2O3* B2O3 Al2O3 SiO2 WEIGHT OF EACH OXIDE 56.1 40.3 94.2 62.0 160.0 69.6 102.0 60.1 -------------------------------------------------------------------------------------------- MATERIAL Expan OF EACH OXIDE 0.15 0.03 0.33 0.39 0.13 0.03 0.06 0.04 Cost/kg -------------------------------------------------------------------------------------------- ------- CUSTER FELDSPAR.... 41.00 617.10 0.00 0.04 0.02 0.00 0.07 0.47 0.00 GERSTLEY BORATE.... 12.00 133.20 0.04 0.02 0.09 3.19 DOLOMITE........... 7.00 184.00 0.04 0.04 0.00 KAOLIN............. 5.00 258.14 0.02 0.04 0.24 TALC............... 15.00 126.23 0.12 0.16 0.25 SILICA............. 20.00 60.00 0.33 0.19 -------------------------------------------------------------------------------------------- ------- TOTAL 100.00 0.08 0.16 0.04 0.04 0.00 0.09 0.09 1.00 0.47 UNITY FORMULA 0.24 0.50 0.14 0.12 0.00 0.29 0.28 3.18 PER CENT BY WEIGHT 4.60 6.82 4.46 2.54 0.11 6.76 9.73 64.97 Cost/kg 0.47 Si:Al 11.33 SiB:Al 12.35 Expan 6.43
I then produced a glaze recipe of higher expansion by introducing high expansion fluxing oxides at the expense of ones of lower value. I did likewise to make a glaze of lower expansion.
I line blended the two new recipes to produce three intermediate mixtures, and thus a range of expansions. These five glazes were applied to more bisque bars and fired. Calculated M.O.R. figures revealed that further reduction in the expansion was necessary to increase strength to optimum. Later adjustments to restore matteness could then be made while maintaining the calculated expansion.
Next, I calculated another even lower expansion recipe and line blended with the original matte glaze, which now formed the upper expansion end. New bars were glazed, a firing done. You can see the results here.
| Butter Matte Glaze | C10 | C9 | C8 | C7 | C6 | ||
| CUSTER FELDSPAR | 41.00 | 41.00 | |||||
| CALCIUM BORATE | 12.00 | 12.00 | |||||
| DOLOMITE | 7.00 | 7.00 | |||||
| KAOLIN | 22.00 | 5.00 | |||||
| TALC | 9.00 | 15.00 | |||||
| SILICA | 25.00 | 20.00 | |||||
| *CaO | .28 | .24 | |||||
| *MgO | .41 | .50 | |||||
| *K2O | .16 | .14 | |||||
| *Na2O | .14 | .12 | |||||
| *Fe2O3 | .00 | .00 | |||||
| B2O3 | .33 | .28 | |||||
| Al2O3 | .58 | .28 | |||||
| SiO2 | 4.31 | 3.18 | |||||
| EXPANSION | 1.02 | 1.25 | |||||
| STRENGTH PSI Modulus of Rupture: | |||||||
| P580 | 9122 | 5597 | 4797 | 4137 | 4164 | 3811 | |
| H550 | 3955 | 8135 | 6538 | 5329 | 4887 | 3930 | |
Notice that the C10 mix has doubled the strength of the H550 clay, a porous stoneware. Not only this, but a piece of pottery made from this combination would be stronger than a piece made from the porcelain using the same glaze! Incredibly, it would be twice as strong as a piece made from the porcelain using glaze C6, the original.
Sure, C6 looks great on the porcelain, it doesn't even craze. However, it produces a very weak piece of ware! C10, unfortunately, has lost some matteness. Further work using any number of calculation strategies will bring it back while maintaining the low expansion.
What have we learned?
- Most of us probably underestimate the effect of glaze fit on strength.
- Maybe porous stonewares are not so bad after all, if they are matched with a glaze that strengthens them.
- Be careful with porcelains; they require extra effort to match your glazes.
- Calculation is practical, predictable, and easier than you think.
- Just because you cannot see crazing does not mean the glaze fits.
Don't mix up these glazes and expect them to produce optimal strength on your clay. Really, for the very best ware, each clay-glaze combination should be tested. However, it is not really practical to do it this way for every glaze. After one or two series of tests, you can correlate calculated expansion values to the clays you use and calculate after that. It is best to stay away from the very high strengths that you might produce during testing (e.g. C10 on H550). This usually means too much compression, and over time, such pieces could weaken and fail.
Related Information
Links
| Articles |
Demonstrating Glaze Fit Issues to Students
Glaze and body can both be adjusted to solve crazing and shivering problems. This describes a simple project to create body glaze combinations guaranteed to craze and shiver to demonstrate the principles involved. |
| Articles |
Crazing in Stoneware Glazes: Treating the Causes, Not the Symptoms
Band-aid solutions to crazing are often recommended by authors, but these do not get at the root cause of the problem, a thermal expansion mismatch between glaze and body. |
| Articles |
Understanding Thermal Expansion in Ceramic Glazes
Understanding thermal expansion is the key to dealing with crazing or shivering. There is a rich mans and poor mans way to fit glazes, the latter might be better. |
| Articles |
Adjusting Glaze Expansion by Calculation to Solve Shivering
This page demonstrates how you might use INSIGHT software to do calculations that will help you increase the thermal expansion of a glaze while having minimal impact on other properties. |
| Troubles |
Glaze Crazing
Ask the right questions to analyse the real cause of glaze crazing. Do not just treat the symptoms, the real cause is thermal expansion mismatch with the body. |
| Tests |
300F:Ice Water Crazing Test
Ceramic glazes that do not fit the body often do not craze until later. This progressively stresses the fit until failure point, thus giving it a score |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
