| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
A Low Cost Tester of Glaze Melt Fluidity
A One-speed Lab or Studio Slurry Mixer
A Textbook Cone 6 Matte Glaze With Problems
Adjusting Glaze Expansion by Calculation to Solve Shivering
Alberta Slip, 20 Years of Substitution for Albany Slip
An Overview of Ceramic Stains
Are You in Control of Your Production Process?
Are Your Glazes Food Safe or are They Leachable?
Attack on Glass: Corrosion Attack Mechanisms
Ball Milling Glazes, Bodies, Engobes
Binders for Ceramic Bodies
Bringing Out the Big Guns in Craze Control: MgO (G1215U)
Can We Help You Fix a Specific Problem?
Ceramic Glazes Today
Ceramic Material Nomenclature
Ceramic Tile Clay Body Formulation
Changing Our View of Glazes
Chemistry vs. Matrix Blending to Create Glazes from Native Materials
Concentrate on One Good Glaze
Copper Red Glazes
Crazing and Bacteria: Is There a Hazard?
Crazing in Stoneware Glazes: Treating the Causes, Not the Symptoms
Creating a Non-Glaze Ceramic Slip or Engobe
Creating Your Own Budget Glaze
Crystal Glazes: Understanding the Process and Materials
Demonstrating Glaze Fit Issues to Students
Diagnosing a Casting Problem at a Sanitaryware Plant
Drying Ceramics Without Cracks
Duplicating Albany Slip
Duplicating AP Green Fireclay
Electric Hobby Kilns: What You Need to Know
Fighting the Glaze Dragon
Firing Clay Test Bars
Firing: What Happens to Ceramic Ware in a Firing Kiln
First You See It Then You Don't: Raku Glaze Stability
Fixing a glaze that does not stay in suspension
Formulating a body using clays native to your area
Formulating a Clear Glaze Compatible with Chrome-Tin Stains
Formulating a Porcelain
Formulating Ash and Native-Material Glazes
G1214M Cone 5-7 20x5 glossy transparent glaze
G1214W Cone 6 transparent glaze
G1214Z Cone 6 matte glaze
G1916M Cone 06-04 transparent glaze
Getting the Glaze Color You Want: Working With Stains
Glaze and Body Pigments and Stains in the Ceramic Tile Industry
Glaze Chemistry Basics - Formula, Analysis, Mole%, Unity
Glaze chemistry using a frit of approximate analysis
Glaze Recipes: Formulate and Make Your Own Instead
Glaze Types, Formulation and Application in the Tile Industry
Having Your Glaze Tested for Toxic Metal Release
High Gloss Glazes
Hire Us for a 3D Printing Project
How a Material Chemical Analysis is Done
How desktop INSIGHT Deals With Unity, LOI and Formula Weight
How to Find and Test Your Own Native Clays
I have always done it this way!
Inkjet Decoration of Ceramic Tiles
Is Your Fired Ware Safe?
Leaching Cone 6 Glaze Case Study
Limit Formulas and Target Formulas
Low Budget Testing of Ceramic Glazes
Make Your Own Ball Mill Stand
Making Glaze Testing Cones
Monoporosa or Single Fired Wall Tiles
Organic Matter in Clays: Detailed Overview
Outdoor Weather Resistant Ceramics
Painting Glazes Rather Than Dipping or Spraying
Particle Size Distribution of Ceramic Powders
Porcelain Tile, Vitrified Tile
Rationalizing Conflicting Opinions About Plasticity
Ravenscrag Slip is Born
Recylcing Scrap Clay
Reducing the Firing Temperature of a Glaze From Cone 10 to 6
Setting up a Clay Testing Program in Your Company or Studio
Simple Physical Testing of Clays
Single Fire Glazing
Soluble Salts in Minerals: Detailed Overview
Some Keys to Dealing With Firing Cracks
Stoneware Casting Body Recipes
Substituting Cornwall Stone
Super-Refined Terra Sigillata
The Chemistry, Physics and Manufacturing of Glaze Frits
The Effect of Glaze Fit on Fired Ware Strength
The Four Levels on Which to View Ceramic Glazes
The Majolica Earthenware Process
The Potter's Prayer
The Right Chemistry for a Cone 6 Magnesia Matte
The Trials of Being the Only Technical Person in the Club
The Whining Stops Here: A Realistic Look at Clay Bodies
Those Unlabelled Bags and Buckets
Tiles and Mosaics for Potters
Toxicity of Firebricks Used in Ovens
Trafficking in Glaze Recipes
Understanding Ceramic Materials
Understanding Ceramic Oxides
Understanding Glaze Slurry Properties
Understanding the Deflocculation Process in Slip Casting
Understanding the Terra Cotta Slip Casting Recipes In North America
Understanding Thermal Expansion in Ceramic Glazes
Unwanted Crystallization in a Cone 6 Glaze
Using Dextrin, Glycerine and CMC Gum together
Volcanic Ash
What Determines a Glaze's Firing Temperature?
What is a Mole, Checking Out the Mole
What is the Glaze Dragon?
Where do I start in understanding glazes?
Why Textbook Glazes Are So Difficult
Working with children
Deflocculants: A Detailed Overview
Description
A detailed look and what deflocculation is, what the most common types of deflocculants are (there are many) and how they compare in function By Nilo Tozzi
Article
Deflocculation and Flocculation
The particles of an argillaceous material, when suspended in water, behave in two entirely different ways, since the electrostatic charges present on their surface may cause both attraction and repulsion. Normally, in an acid environment, the particles of an argillaceous material are attracted to each other, this state is called "flocculation". In an alkaline environment the particles repulse each other, this state is called "deflocculation".
In the state of deflocculation, the charges on the particles have been neutralized, the particles remain in suspension as single units, with a consequent reduction in viscosity. In the state of flocculation, the particles form three-dimensional groups or structures, due to electrostatic attraction, with a consequent increase in viscosity.
Deflocculants
The term "deflocculant" denotes a substance which, when added to scattered particles in suspension, causes a reduction in apparent viscosity. Deflocculants are substances which prevent flocculation by increasing zeta potential and therefore the repulsive forces between particles.
The mechanisms by which deflocculants act can be enumerated as follows:
- A shift of pH towards basic values by addition of bases or by hydrolysis.
- Substitution of flocculant cations, present in the double layer of clays, with alkaline cations.
- Increase of negative charge on argillaceous particles by adsorption of anions with elevated electric field.
- Addition of a protective colloid.
- Elimination of flocculant ions which might be present in solution, via precipitation or formation of coordination complexes. For example via the following reactions:
- BaCO3 + SO4= = BaSO4 + CO3=
- Na2CO3 + Ca2+ = CaCO3 + 2Na
- BaCO3 + Ca2+ = CaCO3 + Ba2+
Normally deflocculants act via a combination of the above-mentioned mechanisms and can be of either organic or inorganic nature.
Main Deflocculants | |
| Organic | Inorganic |
|---|---|
| Humic acids and derivatives | Sodium and potassium carbonates |
| Alkaline lignosulfonates | Sodium and potassium hydroxides |
| Tannin compounds | Sodium silicates |
| Polyacrylates and acrylic derivatives | Phosphates and polyphosphates |
| Polycarbonates | Sodium and ammonium oxalates |
| Sodium citrate | |
| Gum arabic | |
| Low viscosity Na-CMC | |
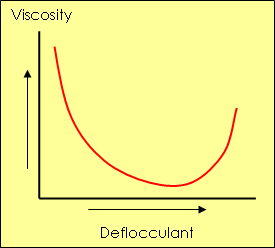
The position of the point of minimum viscosity is affected by slip density
This graph shows that addition of a deflocculating substance causes viscosity reduction to a point at which the forces of attraction are neutralized. At this point, called "full deflocculation", viscosity reaches its minimum value and subsequent additions of deflocculants have an adverse effect.
The most efficient compounds having deflocculant action for uses in ceramics are sodium silicate, polyphosphates (pyro - tripoly - tetrapoly - etc.) and organic sodium and ammonium polyelectrolytes. No single product acts according to all of the mechanisms described above, therefore a mixture of various compounds is usually used, whose combined action is often superior to the sum of their single actions.
For tile pastes, the following products are most commonly used:
- Liquid or solid mixtures of sodium silicates, polyphosphates and polyacrylates
- Sodium tripolyphosphate
- Liquid or solid sodium silicates
Some clays can be easily deflocculated using compounds which raise pH, such as sodium silicate or sodium carbonate, as these contain organic material which can react in the presence of an alkaline environment, forming deflocculant compounds.
Bibliography
P. Prampolini, Ceramica Informazione, 311, 1992, pag.88.
MOST COMMON DEFLOCCULANTS
Sodium carbonate
This compound is commonly called "soda" and has the formula Na2CO3 or Na2CO3.10H2O depending, respectively, on whether it is anhydrous or hydrous. The deflocculating action is carried out by an increase in pH, but the carbonate ion, before hydrolysis, can react with calcium ions that may be present in the solution, thereby forming CaCO3 which is insoluble and therefore a flocculating element is removed from the suspension. This carbonate is often used in combination with a silicate, and the resulting mixture, whose exact proportions have to be arrived at via experimentation, is the traditional fluidifier for fine tableware, porcelain and sanitary fixtures.
Sodium silicate
This is the main deflocculant used for the preparation of pastes for casting or for refractory plastics. The ratio of SiO2 to Na2O can vary from 3.75:1 to 1:1 and is available in liquid or solid form. Sodium silicate increases the pH of the suspension, due to hydrolysis, whereas the silicon separates out in the form of colloidal silica which also performs a role as protective colloid, according to the following reaction:
Na2O.nSiO2 + H2O => nSiO2 + 2Na+ + 2OH
When used alone, the percentage in pastes varies between 0,3 and 0,7%.
Alkaline lignosulfonates
These compounds are water-soluble by-products from the manufacture of cellulose using the bisulphite method. Their molecular mass varies between 200 and 100,000, but the most common types have a molecular mass around 4000 and contain monomers on which 8 - SO3 functional groups can be found, associated with benzene rings. They can also act as binding agents for flocculant cations, but their deflocculant action is carried out by the functional groups already mentioned.
They are anionic polyelectrolytes which are strongly hydrolysed even at pH's below 5 and can be absorbed by argillaceous particles up to a pH of 10. Sure enough, dissociation in sulfonate groups - SO3Na- is considerably stronger than in carboxylic or phenolic groups associated with other polymers of natural origin or resulting from synthesis, such as humates.
Polyphosphates
Alkaline polyphosphates (normally from sodium or ammonium) are dissociated in solution and the anions are absorbed onto the clay particles, generating a strongly negative potential. Moreover they are able to capture polyvalent flocculant cations, such as calcium and magnesium, associated with water and soluble salts.
Polyphosphates evolve slowly, by hydrolysis, and are transformed into orthophosphates, thus reducing their deflocculant power with the aging of suspensions.
The main sodium salts used as deflocculants are listed on the following table.
Polyphosphates | ||
| Name | Formula | Solubility |
|---|---|---|
| Tripolyphosphate | Na5P3O10 | 12% - 140 g/l per 25 C |
| Pyrophosphate | Na4P2O7 | 5% |
| Tetraphosphate | Na6P4O10 | High |
| Esametaphosphate | (NaPO3)6 | Unlimited |
Tripolyphosphate
This is a sodium phosphate triple-polymerised so as to form a single molecule with a chain structure.
Its deflocculant power is shown by an increase in negative charge on the surface of the clay particles, via adsorption of the phosphoric anion, and therefore by an increase of zeta potential which causes repulsion between the particles.
It also forms insoluble compounds with flocculant anions, removing them from the dispersive vehicle and preventing their action. In particular, the tripolyphosphate anion forms complex and highly stable anions with calcium, of type (CaP6O18)4- and (Ca2P6O18)2-.
It hydrolyses in water, increasing pH up to 9-10 depending on its concentration. Products on the market are often a mixture of different salts, mainly anhydrous and hydrous tripolyphosphates with pyrophosphate, metaphosphate and orthophosphate; in some cases there may be residues of reactants used in preparation of the product, such as monosodic phosphate (NaH2PO4) and bisodium phosphate (Na2HPO4). The content of phosphates other than tripolyphosphate must be minimum as these reduce the deflocculant capability of the product.
Tripolyphosphate also exists in two crystalline forms with different speeds of dissolution in water.
Esametaphosphate
As for tripolyphosphate, its deflocculant power is shown by an increase in negative charge on the surface of the clay particles, via adsorption of the phosphoric anion, and therefore by an increase of zeta potential which causes repulsion between the particles. It also forms insoluble compounds with flocculant anions, removing them from the dispersive vehicle and preventing their action. In particular, the tripolyphosphate anion forms complex and highly stable anions with calcium, of type (CaP6O18)4- and (Ca2P6O18)2-.
Alkaline polyacrylates (Na-NH4)
These are polymers of the following type:
- CH2 - CH -
|
COO.Na+ n
Molecular mass varies between 1000 and 20,000.
They are effective deflocculants above pH 5 for the dissociation of carboylic groups and for the absorption of polymeric anions on clay particles.
They are highly stable polymers over time and also under variation of temperature.
They do not interact with plaster moulds and can also be used for hot casting.
They have been used in the traditional ceramics sector since the 1970's.
Polyacrylic acid is obtained from polymerisation of acrylic acid, and after neutralisation with soda or ammonium, sodium and ammonium polyacrylates are obtained. The process allows for adjustment of chain length and it is therefore possible to obtain a broad range of molecular weights, whose value depends on the properties of the product.
Polyacrylates with a molecular weight between 1000 and 10,000 are energetic fluidifiers, whereas those with a weight higher than 10,000 increase viscosity in suspensions. Chains are less rigid and complex than those of CMC and thus products with low molecular weight cause little water retention.
Polyacrylates reduce interactive forces between particles, attaching themselves to those areas of the particles whose charge is responsible for the formation of three-dimensional structures.
Polyacrylates act more strongly than polyphosphates in reducing tixotropy and yield point, and, like them, are strong sequestrators of polyvalent ions.
In case of excessive dosage, yield point can be reduced to zero, in which case sedimentation may occur.
Bibliography
D. Chiavacci, Ceramica Informazione, 355, 1995, p. 593
Polymers with low molecular weight of natural origin or from synthesis The functional groups of those polymers responsible for electrostatic interaction between molecules absorbed on clay particles are essentially:
-COOH; -SO3H; -SO4H; -OH; -NH2-R-COOH
One can list gum arabic, alginates and low molecular weight and low viscosity carboxymethylcellulose, starch derivatives, and vegetal gum and protein derivatives.
Barium carbonate, BaCO3
This is not a true deflocculant, but it aids deflocculation by precipitating the sulphate ion which prevents the process, and is often associated with calcium, magnesium and iron which are flocculant ions.
The anion SO4= is easily absorbed by clay particles, also in substitution of hydroxyls, and prevents reduction of zeta potential.
Barium carbonate is used, even if not very soluble, as it precipitates the sulphate ion easily, whereas barium chloride is soluble but would carry barium ions into the solution which in excess would act as flocculants.
BaCO3 must be added before the deflocculants; quantity varies between 0.02 and 0.1%.
Related Information
Delflocculation accomplishes the seemingly impossible

This picture has its own page with more detail, click here to see it.
It is possible to get that 3000g of porcelain powder into this 1100g of water! And the fluid slurry produced, 2250cc, will fit into the front container. How is this even possible? The water has 11 grams of Darvan 7 deflocculant in it, it causes the clay particles to electrolytically repel each other! An awareness of “this magic” can help give you the determination to master deflocculation, the key enabler of the slip casting process. Determination? Yes, the process is fragile, you must develop the ability to “discover” the right amount of Darvan for your clay mix and water supply. And the ability to recognize what is wrong with a slurry that is not working (too much or little water, too much or little deflocculant).
Links
| Glossary |
Deflocculation
Deflocculation is the magic behind the ceramic casting process, it enables slurries having impossibly low water contents and ware having amazingly low drying shrinkage |
| Glossary |
Zeta Potential
|
| Articles |
Understanding the Deflocculation Process in Slip Casting
Understanding the magic of deflocculation and how to measure specific gravity and viscosity, and how to interpret the results of these tests to adjust the slip, these are the key to controlling a casting process. |
| Typecodes |
Electrolyte
Materials used to control slurry properties of glazes and slips (vicosity, specific gravity). |
By Nilo Tozzi
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
