| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
A Low Cost Tester of Glaze Melt Fluidity
A One-speed Lab or Studio Slurry Mixer
A Textbook Cone 6 Matte Glaze With Problems
Adjusting Glaze Expansion by Calculation to Solve Shivering
Alberta Slip, 20 Years of Substitution for Albany Slip
An Overview of Ceramic Stains
Are You in Control of Your Production Process?
Are Your Glazes Food Safe or are They Leachable?
Attack on Glass: Corrosion Attack Mechanisms
Ball Milling Glazes, Bodies, Engobes
Binders for Ceramic Bodies
Bringing Out the Big Guns in Craze Control: MgO (G1215U)
Can We Help You Fix a Specific Problem?
Ceramic Glazes Today
Ceramic Material Nomenclature
Ceramic Tile Clay Body Formulation
Changing Our View of Glazes
Chemistry vs. Matrix Blending to Create Glazes from Native Materials
Concentrate on One Good Glaze
Crazing and Bacteria: Is There a Hazard?
Crazing in Stoneware Glazes: Treating the Causes, Not the Symptoms
Creating a Non-Glaze Ceramic Slip or Engobe
Creating Your Own Budget Glaze
Crystal Glazes: Understanding the Process and Materials
Deflocculants: A Detailed Overview
Demonstrating Glaze Fit Issues to Students
Diagnosing a Casting Problem at a Sanitaryware Plant
Drying Ceramics Without Cracks
Duplicating Albany Slip
Duplicating AP Green Fireclay
Electric Hobby Kilns: What You Need to Know
Fighting the Glaze Dragon
Firing Clay Test Bars
Firing: What Happens to Ceramic Ware in a Firing Kiln
First You See It Then You Don't: Raku Glaze Stability
Fixing a glaze that does not stay in suspension
Formulating a body using clays native to your area
Formulating a Clear Glaze Compatible with Chrome-Tin Stains
Formulating a Porcelain
Formulating Ash and Native-Material Glazes
G1214M Cone 5-7 20x5 glossy transparent glaze
G1214W Cone 6 transparent glaze
G1214Z Cone 6 matte glaze
G1916M Cone 06-04 transparent glaze
Getting the Glaze Color You Want: Working With Stains
Glaze and Body Pigments and Stains in the Ceramic Tile Industry
Glaze Chemistry Basics - Formula, Analysis, Mole%, Unity
Glaze chemistry using a frit of approximate analysis
Glaze Recipes: Formulate and Make Your Own Instead
Glaze Types, Formulation and Application in the Tile Industry
Having Your Glaze Tested for Toxic Metal Release
High Gloss Glazes
Hire Us for a 3D Printing Project
How a Material Chemical Analysis is Done
How desktop INSIGHT Deals With Unity, LOI and Formula Weight
How to Find and Test Your Own Native Clays
I have always done it this way!
Inkjet Decoration of Ceramic Tiles
Is Your Fired Ware Safe?
Leaching Cone 6 Glaze Case Study
Limit Formulas and Target Formulas
Low Budget Testing of Ceramic Glazes
Make Your Own Ball Mill Stand
Making Glaze Testing Cones
Monoporosa or Single Fired Wall Tiles
Organic Matter in Clays: Detailed Overview
Outdoor Weather Resistant Ceramics
Painting Glazes Rather Than Dipping or Spraying
Particle Size Distribution of Ceramic Powders
Porcelain Tile, Vitrified Tile
Rationalizing Conflicting Opinions About Plasticity
Ravenscrag Slip is Born
Recylcing Scrap Clay
Reducing the Firing Temperature of a Glaze From Cone 10 to 6
Setting up a Clay Testing Program in Your Company or Studio
Simple Physical Testing of Clays
Single Fire Glazing
Soluble Salts in Minerals: Detailed Overview
Some Keys to Dealing With Firing Cracks
Stoneware Casting Body Recipes
Substituting Cornwall Stone
Super-Refined Terra Sigillata
The Chemistry, Physics and Manufacturing of Glaze Frits
The Effect of Glaze Fit on Fired Ware Strength
The Four Levels on Which to View Ceramic Glazes
The Majolica Earthenware Process
The Potter's Prayer
The Right Chemistry for a Cone 6 Magnesia Matte
The Trials of Being the Only Technical Person in the Club
The Whining Stops Here: A Realistic Look at Clay Bodies
Those Unlabelled Bags and Buckets
Tiles and Mosaics for Potters
Toxicity of Firebricks Used in Ovens
Trafficking in Glaze Recipes
Understanding Ceramic Materials
Understanding Ceramic Oxides
Understanding Glaze Slurry Properties
Understanding the Deflocculation Process in Slip Casting
Understanding the Terra Cotta Slip Casting Recipes In North America
Understanding Thermal Expansion in Ceramic Glazes
Unwanted Crystallization in a Cone 6 Glaze
Using Dextrin, Glycerine and CMC Gum together
Volcanic Ash
What Determines a Glaze's Firing Temperature?
What is a Mole, Checking Out the Mole
What is the Glaze Dragon?
Where do I start in understanding glazes?
Why Textbook Glazes Are So Difficult
Working with children
Copper Red Glazes
Description
A study of the mechanism behind the color in color red glazes by Karl Platt.
Article
A report to the Clayart discussion group by Karl P. Platt kplatt@glass.com
Red Copper (Cu) glazes are distinctive and have been highly prized in history - everyone's heard about the Chinese guy who died taking the "secret" to the grave with him, leaving the Emperor quite disgruntled.
Cu red glazes are based on adding Cu into the glaze as an oxide and then exposing it to a reducing firing. If a sample of the glaze is drawn from the kiln at full heat should show at most a light straw color and it may turn red on cooling. The red is produced on cooling by crystals that come out of solution with the glaze. The composition of these crystals that has been the source of controversy.
Up until 1960 or so it was held that the color was due to metallic copper crystals. Really, this was accepted as being quite obvious. Then came Atamaram and Prasad, who suggested that the red color was actually due to Cu2O (red copper oxide) crystals.
Atamaram and Prasad's paper makes for very interesting reading on a number of levels - I wish I had a copy of it here! Recognizing the difficulties had in making Cu Red, and the desirability of the color as used in glass bangles Indian women especially like, they set out to study and refine the parameters of Cu red development. In the course of their work they came to the Cu2O conclusion, but their work was criticized because they added large amounts of Cu (up to 5 wt%) to their
glasses.
However, through their work they did obtain delicious and repeatable red glasses.
Behind Atamaram and Prasad came Rawson who showed that the color of Cu red was consistent with the results expected from what is known as the Mie Scattering Theory for Cu-metal. Mie theory predicts what wavelengths will be preferentially reflected from the metal surface. It's real complicated and we'll leave it to say only that the red Rawson found by measuring the spectrum of the red in his glasses gave results that were consistent the presence of Cu-metal.
Amal Paul, a guy no-one can say enough about, undertook to sort out the controversy about just what it was that made reduced Cu glasses red - Cu2O, Cu-metal or a mix if the two. He did his studies in a glass made of 30 Na2O and 70 B2O3 --- this does not represent either a useful glass or glaze, but it is easy to melt. No tin was added to the glass and the amount of Cu this glass will hold is very low-up to 0.13wt% Cu taken as metal.
Paul concluded that both Cu and Cu2O are present and the "better"-more pure - Cu reds were abundant in Cu2O.
Cu belongs to a group of metals known as the Nobel Metals. The Nobel Metals are Cu, Ag and Au. In this order they are progressively less likely to form oxides.
Recalling our discussion on Redox, [Editor: a discussion on Clayart about the mechanisms of oxidation and reduction] we can say that the outer electrons on these metals are progressively more rigidly held moving from copper to gold. Gold stays reduced, silver resists oxidation, and copper will go along and ditch an electron or two depending on the crowd it's in.
Cu oxides, of course, are well known. You can buy Ag2O from the chemical house, but apart from that it's not seen much - the film that develops on your Ag tableware is not predominantly oxide. Au oxide is never encountered in normal circumstances; this is the source of value in gold.
When Ag or Au are added to a glaze, it is not necessary to employ heavy reduction to produce metal atoms - Ag and Au would rather be metal atoms. There is a limit as to how many of these metal atoms can be in solution (colorless) with the glaze - naturally this limit is called the solubility limit. When this critical limit is exceeded, the excess metal atoms combine with their kind to form crystals.
These crystals of Ag or Au metal produce color in the glaze.
The color is determined by how these crystals are shaped, how they are distributed in the glaze/glass and their size. The number of metal atoms that can be held in solution with the glaze decreases as temperature decreases.
As the glaze cools metal crystals will develop -- Often tending toward being fewer and larger where big crystals form at the expense of little ones. However, the little crystals can also coagulate, and this affects the color observed.
The size of these crystals is on the order of 50-200 millimicrons -- +/- a little. If the crystals get large the glass looks like liver looks (tastes?) and is called livery. If you made livery glass/glaze, you blew it. Do not pass Go. No Doughnut. Call the dumpster man. We're not here to make livery rubies.
The color in glazes containing nobel metal crystals is mainly produced by the absorption of light by the metal crystals. Scattering effects have no role in producing the color unless the glass is livery. Big crystals scatter enough light to make the glaze appear opaque in reflected light and (densely) colored in transmitted light. We almost always look at rather than through glazes, so it is the reflected results which matter to us.
Crystallization occurs within a limited range of temperatures. Above some temperature there's too much thermal agitation to allow the metals to organize into crystals, and below some temperature the glaze will be too viscous to allow atoms to migrate towards a developing crystal. Hold this notion, it'll appear again.
Au (gold ruby) is very rarely used in Studio Ceramics. I can't think of anyone hand-rolling their own Au reds - if you're out there please stand-up.
There are, however, Au ruby overglazes and glass enamels commercially available. Most of these are based on soft fritted lead glass and they're not cheap - not because they have huge amounts of Au in them (there are very tiny amounts), but they're tough to make. There's nothing to preclude anyone from making Au ruby glazes except that errors are a little pricey in terms of time and providing for precision. In terms of cash cost, it's really not so terrible as the amount of gold needed is very small.
Au glass/glaze has its distinctive ruby color. Fenton's sells as "Cranberry" Glass. Apart from tableware Au red is often seen in colored sheet glass. There are (really beautiful) blue Au glasses which form when the conditions cause the Au crystals to become large-ish and very non-spherical.
Silver (Ag) can make almost any color in glaze if you know how to manipulate it, but usually it gives yellow. Ag is essentially never used in modern art Ceramics, but the ancients used it widely - especially the Persians who developed fantastic lustres after the collapse of Rome -- the artisans had to go somewhere.
By applying a paste of AgNO3, Kaolin and a little BaSO4 on the surface of bright soda-rich glazes and then refiring the pot to 1400 F/650C or so you can "stain" the glaze locally to nice effect.
Glassmakers use Ag quite often to develop a number of effects. These range from yellow glass to brown glass to a glass that is multicolored in reflected light, but yellow/amber in transmitted light. There are also silver glasses that develop a metallic sheen with reduction.
Essentially spherical crystals of Ag metal cause the yellow and brown glasses. If the color is non-uniform, the stuff the Ag is in is probably not uniform. When the glass turns brown it is also frequently turbid (milky). This is owing to having formed large and numerous crystals - scattering of incident light, and mushy absorption characteristics tending to longer (more red) wavelengths.
Multicolored effects seen in silver glasses/glazes are due to the development of non-spherical crystals. The mechanics of all of this are not, however, our concern here, but Peet mentioned these and I thought it would be worth a brief mention as these effects could also be developed in glazes as well.
Silver glazes have fantastic potential that is overlooked in Studio Ceramics. It is possible to produce any color in a very pure form using only silver. Accomplishing this is a delicate dance, but like Tango, the logic is clear. If you've managed to come to here without hitting the delete key, I'd like to solicit a few collaborators in elaborating Ag in decorative glazes.
But we're really interested in Cu-reds why all this about silver and gold? Well, the formation of Cu-reds follows the same lines in terms of the crystallization mechanics - oversaturation --> crystal formation --> growth or coagulation of the crystals. However, in the case of Cu it's not simply a matter of precipitating metal.
Cu can do a number of things when added to a glaze. In alkaline glazes it yields a very distinct blue color (Cu2O). In less alkaline glazes it's green. (CuO). This indicates the importance of composition on color development. Red, however, can be produced in almost any base glass.
In fact, Cu is quite sensitive to its chemical environment and I've found it to be a good and very handy indicator as to the acidity or basicity of a glaze - kind of a litmus test. Simply, if Cu is the only colorant in a glaze, and the glaze is fired in air, how blue it is gives a rough idea as to what you can expect from the glaze in terms of its many other chemistry sensitive colors.
You can't simply chuck Cu-oxide into any 'ole glaze and expect it to come out red. The following factors come into play:
- Composition
- The presence of tin oxide
- Reduction
- Cooling and sometimes reheating
In terms of composition, the glaze needs to be able to support the solution of Cu. To achieve this it needs to have things in it that are friendly to the presence of metal. The best of these is PbO - I can hear the gasps of horror now. Yes, that Godzilla of the Elements. Bismuth is another good option in some glazes, Zn helps and there is, of course, tin (Sn).
Sn does a couple things. First it improves the solubility of Cu. Metals, per se, aren't really very soluble in glaze and if you can't get the metal dissolved, it can't very well be precipitated in any organized fashion. Second, on cooling, Cu tends to attract Sn atoms from the glaze. These atoms sort of "coat" the crystals as they are developed and thus serves to control their size by limiting the attachment of further Cu atoms to the crystal. This behavior is that of a protective colloid and it is of great advantage. Because if the crystals get big, the glaze turns "livery" looking, and the doughnut remains elusive. Third, to the
extent that Sn has limited solubility in SiO2 or B2O3 based glassy material, it probably also serves to provide nuclei on which the coloring crystals can grow.
Tin oxide is added to all practical non-lead Cu red glazes in amount way beyond what's necessary to promote good solution of Cu in the glaze - many compositions contain up to 4-wt%. Of course, if there's too much tin it doesn't all dissolve -- causing opacity. This may or may not be desirable.
Tin is volatile at high temperatures and a lot of it leaves the very thin glaze film by evaporation. Compensating this evaporation is important to how much tin will remain dissolved in the glaze. This explains the large amounts of SnO2 in many reported glaze compositions. Some kilns have turbulent atmospheres and a larger amount of evaporation would be anticipated in these circumstances. In glassmaking, if you melt in a covered pot, in which evaporation is not an issue (in most cases), the amounts of SnO2 required seldom exceed 2 wt%.
If you use too little Sn to promote the solution, Cu will precipitate on the spot with dreadful results. This is one of the reasons that application of the glaze is so important and why really thin films often fail to develop a nice red - when red color forms at all in a tin depleted glaze, it often has the color of liver instead of a crisp red.
The amount of Cu necessary to develop a good red depends on how much of it can be dissolved. This depends on how much the glaze would dissolve on its own and how much this is improved by the presence of Sn. Many pottery glazes contain what I feel is a lot of Cu-oxide in the batch, but that's just an opinion. The best reds always contain the least amount of Cu. Reduction is the critical step in producing a nice Cu red.
Amal Paul's 30 Na2O 70 B2O3 glass was melted in a little electric furnace with a strictly controlled reducing atmosphere - CO-CO2 mixtures metered into the furnace with precision gear. He found that as the "amount" of reduction increased there is a level below which no red forms; a (point) range of reduction within which good reds developed and a point (range) above which the color is funky.
His conclusions were:
In reduction one wants to achieve an equilibrium which includes only Cu2O (red copper oxide) and Cu-metal, with no CuO present. The amount of Cu-metal dissolved in the glaze is fixed by the glaze composition and the amount of Cu2O present in the glaze is fixed by the atmosphere. It should be mentioned that by using the term Cu2O it is not meant that molecules of Cu2O are floating about in the glaze. On the contrary, it means that Cu+1 ions are in the glaze and that on cooling Cu2O crystals (which are red) are formed. There is some degree of reduction at which Cu2O solubility is at a maximum. This is the point you want to find.
If the reduction applied to the glaze is too weak CuO forms together with Cu2O and Cu-metal. This is not where you want to be at all because at this level of reduction, the glaze will be unsaturated in Cu2O and Cu-metal. As a result nothing will crystallize and you won't see any red. However, owing to poor mixing of the glaze batch it frequently happens that red patches develop in the presence of green CuO. This can be a nice effect.
If reduction is too strong an abundance of Cu metal is formed at the expense of Cu2O, the glaze will precipitate Cu-metal on the spot, as its solubility will have been exceeded, and the color is murky. This is an important distinction between Cu red glazes and Cu red glasses. In an over-reduced glass the metal comes out of solution and sinks to the bottom of the crucible - often forming little beads that drill holes in the crucible. In glaze, the Cu metal can't wander away. Sometimes it manages to oxidize again depending on the firing conditions and it can also form a metallic film on the surface of the finished glaze. The extreme character of this behavior is widely exploited in raku.
The thermodynamic considerations of all of this are tedious, and it's not worth going into all of it here, but the sum results are:
In reduction you can produce three forms of Cu in the glaze. These are:
CuO, Cu2O and Cu-metal.
The ideal degree of reduction will be a little different for each base glaze. I don't have time to crunch the numbers to give some precise fuel/air mix, but if someone else wants to go through it, feel free. In a little more absolute terms, you want to produce an oxygen pressure somewhere between 10^-12 and 10^-15 atmospheres. This corresponds to a CO/CO2 ratio around 1/10^5 - I pulled these numbers off of an Ellingham diagram, they were not calculated.
Of course, rich combustion yields Hydrogen, too, and this needs to be considered. Also, Ellingham diagrams say nothing about the "activity" of the metal/oxide in glaze and these effects can be profound.
Apart from applying heavy science to making Cu red glazes we all know that arriving at the best fuel/air ratio can, of course, be derived by trial and error - as has been done for millennia.
This takes us to the importance of having a stable combustion system with means for metering the amount of fuel and air entering the kiln. Precise combustion is elusive in natural draft kilns subject to wind, variable atmospheric pressure and so on. It can be achieved by experienced operators constantly tending the fires. High-pressure gas "venturi" burners will furnish better repeatability in natural draft kilns in all circumstances. Forced air combustion can be metered very precisely by metering orifices.
We should note again that oxygen analyzers cannot meter reduction - they are sensitive to the presence, not the absence of oxygen. As such other means, like metering orifices, or even a decent pressure gauge are preferred.
We've established that there is some degree of reduction at which the amount of Cu2O is at a maximum and that this is where we will get the best color. Establishing this condition in the glaze can be done several ways. Some like to begin reduction early in the firing - around 1700 F or so and maintain this degree of reduction through the end of the firing. This is fine. Others like to reduce the pi$$ out of the kiln for a short time at high temperature. This can work, too, but it never goes as well as the former approach.
In very practical terms, a glaze film is really thin - usually way less than a millimeter. While glaze is usually pretty viscous stuff, it
doesn't take terribly long at high temperatures for equilibrium between Cu/Cu2O and the atmosphere to be established. I'd suggest that firing in neutral conditions until the last couple or three hours of the firing, and then adding the necessary reduction will be a more economical approach to obtaining the best results. Three hours is plenty long enough to establish equilibrium. Moreover, really prolonged reduction has other affects which may not be desirable at all - like depleting the glaze surface of Na, messing with the body color in undesirable ways, deteriorating the kiln's refractories, and so on.
Re-oxidizing, as it were, can occur if the circumstances are right. Avoiding this can be done by cooling rapidly to 1,700 F or so. Maintaining reduction during cooling is sometimes necessary to control the redox balance in the glaze.
We know that the color is formed by the precipitation of Cu and Cu2O crystals on cooling. Usually a kiln will cool slowly enough so that these crystals have plenty of time to form in the natural cooling of the kiln -- net cooling rates of 1-3 degrees F/minute are common. Cooling will be faster at first and then proceed at a progressively slower rate owing to the diminished temperature gradient between the interior of the kiln and the air.
There will be some temperature at which the crystallization rate is highest. Above this temperature the thermal agitation within the glaze is too great to permit crystals to organize and below it the viscosity of the glaze retards the progress of Cu or Cu+1 to developing crystals.
There will also be some temperature at which the precipitation of Cu2O is at a maximum on cooling. If you find that temperature and hold it for a spell you get better reds.
There may be the weird instance where cooling was too fast and no color appears. In such a case one can reheat the pot to 1,600-1,800 F and the red will form - assuming you reduced correctly.
The presence of P2O5 lends to ruby formation and this was well known to potters in early civilizations. It has a reducing influence by its presence and it is highly insoluble in the host glaze. As a result P2O5 rich droplet regions will form within the glaze and these will promote development of the red crystals. An excess of P2O5 gives opalesence - this can be beautiful.
It would be useful to assemble and examine studio results a lot more carefully. There's a great deal of experimentation in the archives of the last 25 years we could probably learn something from.
Getting the quantities of Sn and Cu right is something that can be worked out using line-blend methods. Any well made shiny glaze will serve as a host for Cu Red. The maximum Sn and Cu you'd want to use are around 3%. Excellent Cu reds have been made with a lot less of both elements. Sn is typically add to the batch in larger amounts than Cu - 3:1 is a frequent ratio. The amount of Cu is fixed its solubility in the glaze - you want to use just enough. The minimum amount of Sn is that
necessary to evade having it all evaporate.
The amount of Cu that crystallizes depends strictly on it's concentration. Less is usually more in Cu reds.
Someone else might have the patience to elaborate how this line-blend should go together. I'd do it on a triaxial in 5 divisions (20% increments) with the corners being: Neat glaze, 3% Cu, 3% Sn.
Make enough glaze to make 3 or 4 test tiles (of the same clay body) of each mixture and fire these in separate firings in the same place in the kiln. This'll give you an idea what the quality of the firing is like - assuming you made homogeneous glaze in the first place.
Adding SiC to the glaze to furnish reduction in electric kilns gives results ranging from moonscape to colorless glaze. In the main, it doesn't work real well....at all. You can also toss organic material into the kiln - I've always been amused by the use of mothballs - they work, but the stench.... Charcoal is a good alternative. You could, as well, be really anal and go out and get tanks of CO and CO2 to inject into the kiln. Talk to your welding gas supplier. And if you do this stuff, don't do it in a confined space.
Remember to do an oxidizing firing after reducing in the electric kiln to build up the Al2O3 film on your Kanthal elements. Nonetheless, element life will be diminished by reduction firings.
Elemental Si is something that should be tried as an in-glaze reducing agent. Maybe someone's already done it. It's cheap, widely available - especially to those who live near a steel works - and very potent in its effects. The advantages of Si as a reducing agent are several fold, but the big one it that you can melt with more correct combustion, and a few grams of Si has always been a lot cheaper than 6 or 7 hours of bad combustion.
Alright, I don't know about you, but I've had quite enough of all of this.
Related Information
Copper red reduction glaze at cone 9 reduction
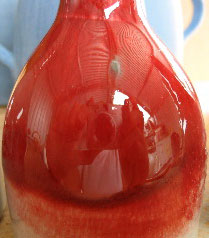
This picture has its own page with more detail, click here to see it.
The color red is difficult to achieve in ceramics. In reduction firing copper can turn many dazzling shades of red.
Copper can produce bright red glazes in correct reduction firing

This picture has its own page with more detail, click here to see it.
Links
By Karl Platt
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
