| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Ferro Frit 3249
Alternate Names: F3249 Frit, F3249 (Ferro)
Description: Low expansion leadless magnesia borosilicate frit
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 3.50% | 0.17 | |
| MgO | 12.20% | 0.83 | |
| B2O3 | 28.90% | 1.14 | |
| Al2O3 | 13.30% | 0.36 | |
| SiO2 | 42.10% | 1.92 | |
| Oxide Weight | 273.88 | ||
| Formula Weight | 273.88 | ||
Notes
No potter or manufacturer should be without this type of frit (despite how expensive it is). Every frit manufacturer will be making a magnesia borosilicate like this (because it is needed to lower the thermal expansion of glazes and glasses).
Frit 3249, from Ferro (now Vibrantz), is an example of this type. It is valuable because it introduces a form of MgO (the lowest expansion flux) that will melt at much lower temperatures than MgO-sourcing raw materials like dolomite or talc (trading some of the high-expansion fluxes in a glaze for MgO is the most effective way to reduce glaze thermal expansion). Also, high MgO is the mechanism of some of the best matte glazes and a frit like this is often the best way to source it.
Ferro specifies this as a bonding agent for grinding wheels. However, frits are sources of oxides, if one supplies the oxides we want and melts well, then it is fine regardless of its label. The process of working this frit into a recipe, to supply some or all of the MgO, is among the most fascinating demonstrations of glaze chemistry. The chemistry of Frit 3249 can be inconvenient at times because it can often oversupply the B2O3 if being used to supply all of the MgO needed.
Fusion frit F-69 has the same chemistry as this, they label theirs a "ceramic frit" (and it has the lowest expansion of any they make). Most other frit manufacturers also make a frit of similar chemistry.
There is some question about how well Ferro maintains the chemistry of this product (and thus whether it is suitable for ceramics). Some users have found variations in the surface quality of their glazes using this. That said, we have found this one to be less soluble than the equivalent Fusion frit (F-69). And far less expensive.
This frit does present an anomaly concerning its use as a source of MgO (instead of raw materials like dolomite and talc). While this generally works well in transparent and stained glazes, producing better clarity and melting, it also seems to attack the aesthetic mechanisms of some reactive glazes that rely on rutile. It is not completely clear why.
Related Information
These common Ferro frits have distinct uses in traditional ceramics

This picture has its own page with more detail, click here to see it.
I used Veegum to form 10 gram GBMF test balls and fired them at cone 08 (1700F). Frits melt really well, they do have an LOI like raw materials. These contain boron (B2O3), it is a low expansion super-melter that raw materials don’t have. Frit 3124 (glossy) and 3195 (silky matte) are balanced-chemistry bases (just add 10-15% kaolin for a cone 04 glaze, or more silica+kaolin to go higher). Consider Frit 3110 a man-made low-Al2O3 super feldspar. Its high-sodium makes it high thermal expansion. It works really well in bodies and is great to make glazes that craze. The high-MgO Frit 3249 (made for the abrasives industry) has a very-low expansion, it is great for fixing crazing glazes. Frit 3134 is similar to 3124 but without Al2O3. Use it where the glaze does not need more Al2O3 (e.g. already has enough clay). It is no accident that these are used by potters in North America, they complement each other well (equivalents are made around the world by others). The Gerstley Borate is a natural source of boron (with issues frits do not have).
Frits do not dissolve in water, right? Wrong.

This picture has its own page with more detail, click here to see it.
This is an example of two types of crystals that have formed on the surface of a fritted glaze after a long period of storage (Ferro Frit 3249 in this case). Frits are formulated to give chemistries that natural materials cannot supply. To do that they have to push the boundaries of stability (solubility). Any frit that has an inordinately high amount (compared to natural sources) of a specific oxide (in this case MgO) or lacks Al2O3 (like Frit 3134) are suspect.
Matte cone 6 glazes have identical chemistry but one melts more. Why?

This picture has its own page with more detail, click here to see it.
These are 10 gram GBMF test balls that we melted on porcelain tiles at cone 4 (top two) and cone 6 (bottom two). They compare the melt fluidity of G2934 (left) and G2934Y (right). The Y version sources its MgO from frit and talc (rather than dolomite). It is a much more fluid melt because the frit is yielding the oxides more readily. But Y has a key benefit: It has a much lower LOI, producing fewer entrained air bubbles and therefore fewer surface defects. And, even though it runs much more, it has the same matte surface! As long as it is applied at normal thickness, the extra melt fluidity does not cause any running. And it has another benefit: Less cutlery marking issues. It is actually a very durable and practical food surface glaze, having a low thermal expansion that fits almost any body. Although these appear glossy here, on ware they have the identical pleasant silky matte surface.
Ferro Frit 3249 vs Fusion F-69 at cone 04

This picture has its own page with more detail, click here to see it.
The chemistry of these two supposedly interchangeable frits is very similar (the difference being that 3249 has 3% CaO that is missing in F-69). But that does not appear to account for this difference in melt fluidity at cone 04! However, as temperatures increase 3249 rapidly becomes more active. Inspite of the difference here we have found the two work interchangeablely in our G2934Y recipe. Obviously, the F-69 is going to make glazes melt better, at least at low fire.
Frits work much better in glaze chemistry

This picture has its own page with more detail, click here to see it.
The same glaze with MgO sourced from a frit (left) and from talc (right). The glaze is 1215U. Notice how much more the fritted one melts, even though they have the same chemistry. Frits are predictable when using glaze chemistry, it is more absolute and less relative. Mineral sources of oxides impose their own melting patterns and when one is substituted for another to supply an oxide in a glaze a different system with its own relative chemistry is entered. But when changing form one frit to another to supply an oxide or set of oxides, the melting properties stay within the same system and are predictable.
Crystallization of Rutile at cone 6 completely subdued? How?

This picture has its own page with more detail, click here to see it.
These glazes are both 80% Alberta Slip, but the one on the right employs 20% Ferro Frit 3249 accelerate the melting (whereas the left one has 20% Frit 3134). Even though Frit 3249 is higher in boron and should melt better, its high MgO stiffens the glaze melt denying the mobility needed for the crystal growth.
A fritted source of MgO has sabotaged the visual character of this glaze

This picture has its own page with more detail, click here to see it.
This is G2917 Ravenscrag floating blue and G2917A. The latter was also supposed to be floating blue. Both are descendants of the original G2826R floating blue, preserving its chemistry but sourcing it from more user-friendly materials. The glaze on the right takes the mechanisms of the other two and compromises the chemistry in the direction of lowering the thermal expansion (to reduce crazing). The use of high-MgO frit 3249 is part of the strategy (instead of sourcing it from talc or dolomite). But this has completely killed the visual! The solution will be to return to the use of talc to source the MgO.
G1215U vs. G1215W glaze flow test

This picture has its own page with more detail, click here to see it.
These recipes have the same chemistry but the 1215U uses frit to source the MgO and CaO. This demonstrates that it is not just chemistry that determines melt flow. Raw materials are crystalline and have different melting patterns than frits (which have already been melted and reground).
Low expansion version of cone 6 Alberta Slip amber glaze glaze

This picture has its own page with more detail, click here to see it.
Alberta Slip with 20% added frit 3134 (left) fired to cone 6 on a porcelain. This is the standard GA6-A recipe. On the right 20% frit 3249 has been used instead. That is a low expansion frit so if you have crazing with the standard recipe, consider trying this one.
How to adjust the G1916Q low fire clear glaze when it crazes

This picture has its own page with more detail, click here to see it.
This is Plainsman Buffstone, fired at cone 04. The piece emerged from the kiln without crazing. The mug was heated to 300F and plunged into ice water (the 300F-to-ice-water IWCT test). This is what happened. Water is being absorbed into the porous body through the craze lines. This is G1916J, a variation on the G1916Q recipe. J is just two materials, 85% Ferro Frit 3195 and 15% EPK. What recipe adjustment is needed? Substitute some of the Frit 3195 for low expansion Frit 3249. We have found that a 55:30:15 of 3195:3249:EPK recipe will work. Since both 3195 and 3249 melt transparent at cone 04, blending them together does not change the appearance (actually 3249 is glossier and actually improves the surface).
How I calculated a feldspar-to-frit replacement in a cone 10R clear glaze

This picture has its own page with more detail, click here to see it.
A screen shot of side-by-side panels in my account at Insight-live.com. On the left is the original G1947U recipe. On the right I have substituted frit 3110 as a higher-concentration-source of KNaO. This enabled actually increasing KNaO to get a better gloss and melt. I have introduced calcined kaolin (to ratio with the raw kaolin to control slurry and drying properties). I added frit 3249 to introduce low-expansion MgO to counterbalance the higher levels of KNaO. That frit, like the 3110, will not only melt things better simply because it itself was pre-melted, but it also brings along a little boron. That supercharges melting and enables an enhancement: The addition of more SiO2. The low thermal expansions of MgO, SiO2 and B2O3 counterbalance the increase that will occur as a result of the higher KNaO (0.15-0.26). Sound boring? When you see the unexpected results you might think differently (see the linked post below). I never considered using frits at cone 10R before, this success led to an improvement in my main silky matte glaze also.
GA6A Alberta Slip base using Frit 3124, 3249 and 3195 on dark body

This picture has its own page with more detail, click here to see it.
The body is dark brown burning Plainsman M390 (cone 6). The amber colored glaze is 80% Alberta Slip (raw:calcine mix) with 20% of each frit. The white engobe, L3954B, on the inside of two of the mugs is L3954A (those mugs are glazed inside using transparent G2926B). The Alberta Slip amber gloss glaze produces an ultra-gloss surface of high quality on mugs 2 and 3 (Frit 3249 and 3195). On the outside we see it this glaze on the white slip until midway down, then on the bare red clay. The amber glaze on the first mug (with Frit 3124) has a pebbly surface. These are fired using a drop-and-soak firing schedule. Some caution is required with the 3249 version, it has low thermal expansion (that is good on bodies that normally craze glazes, but risks shivering on ones that do not).
GA6A Alberta Slip base using Frit 3249 and 3195 on buff body

This picture has its own page with more detail, click here to see it.
The body is buff burning Plainsman M340 (cone 6). The amber colored glaze is 80% Alberta Slip (raw:calcine mix) with 20% of each frit. The white engobe on the inside of mug 1 is L3954A (also glazed inside using transparent G2926B). These frits are producing an amber gloss glaze of high quality. On the outside of mug 1 we see the 3195 version on the white slip until midway down, then on the bare buff clay (the other has the 3249 version). These mugs are fired using a drop-and-soak firing schedule. A couple of caveats: Frit 3249 has a very low thermal expansion, use it on bodies that craze other glazes (like Plainsman P300), it could shiver on stonewares like this. Both of these frits prevent the formation of bloating blues (with additions of rutile or titanium).
P300 and M370 mugs with GA6A Alberta Slip (using Frit 3249)

This picture has its own page with more detail, click here to see it.
Rather than the normal 80:20 AlbertaSlip:Frit3134 recipe, this one substitutes Frit 3249 (super low expansion). The glaze is less runny and even glossier on these Plainsman porcelains. They are fired at cone 6 in a cool-and-soak firing. They survived an BWIW test (boiling water:ice water) without crazing (likely because of the low expansion of frit 3249). The finish is dazzling, a brilliant amber glass with no defects and perfectly even coverage. Of course, the iron in the glass prevents the colors of the blue underglaze from showing through. But the black is great.
Devitrification of a transparent glaze

This picture has its own page with more detail, click here to see it.
This glaze consists of micro fine silica, calcined EP kaolin, Ferro Frit 3249 MgO frit, and Ferro Frit 3134. It has been ball milled for 1, 3, and 6 hours with these same results. Notice the crystallization that is occurring. This is likely a product of the MgO in the Frit 3249. This high boron frit introduces it in a far more mobile and fluid state than would talc or dolomite and MgO is a matting agent (by virtue of the micro crystallization it can produce). The fluid melt and the fine silica further enhance the effect.
Five frits. One kiln at 1850F. Big chemistry drama.

This picture has its own page with more detail, click here to see it.
Five common North American Ferro Frits fired at 1850F on alumina tiles (each started as a 10-gram GBMF test ball and flattened during the firing). At this temperature, the differences are more evident than at 1950F. The degree of melting corresponds mainly, but not only, to the percentage of B2O3 present. Frit 3134 is the runaway leader because it couples that with almost no Al2O3 to stabilize the melt. Notice also the crack pattern, it is also very high in Na2O and thus has a high COE. However, why does Frit 3110 melt so well even though it contains almost no B2O3? Even higher Na2O, it is a powerful flux. Its COE is even higher than Frit 3134, but it is thick enough to have resisted cracking far (but it will in time).
Melt fluidity comparison - 1750F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1750F and held for 15 minutes. Frit 3110 has taken off. And F75, 3195 and 3134 (the latter two having big differences in surface tension).
Various frits fired at 1950F

This picture has its own page with more detail, click here to see it.
These sixteen GBMF test balls have melted down onto a slab of grogged clay. Kiln fired at 108F/hr for the last 100 degrees F and held for 15 minutes. This demonstrates the comparison value of this test and how various frits compare in their melting character.
Frits fired to 2050F

This picture has its own page with more detail, click here to see it.
These are higher temperature frits. 10 gram balls were melted on to this tile.
Various frits fired at 1850F

This picture has its own page with more detail, click here to see it.
16 GBMF tests on a slab of grogged clay. Kiln fired at 108F/hr for last 100 degrees F and held for 15 minutes.
1700F Frit Melt-Off: Who is the winner? Not the lead bisilicate!

This picture has its own page with more detail, click here to see it.
These were 10g balls melted using our GBMF test. We fired at a temperature far lower than typical bisque, notice how many of them are already melting well! Frit 3602 is lead bisilicate. But it got "smoked" by the Fusion FZ-16 high-zinc, high-boron zero-alumina! Maybe you always thought lead was the best melter. That it produced the most transparent, crystal-clear glass. But that is not what we see here. That being said, notice the lead is not crazing but the FZ-16 is crazing badly, that is a problem for many applications using this frit, it relies on a high percentage of KNaO. Notice something else: Each frit has a distinctive melt fingerprint that makes it recognizable in tests like this. Want to get some of this frit for pottery? You can't, Fusion Ceramics doesn't want to handle retail sales of smaller quantities.
Frit melt fluidity comparison - 1300F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1300F and held for 15 minutes. Some are still burning off carbon (which seems strange). There are two early leaders: Ferro frit 3110 and Fusion frit F75 are starting to deform (they have almost the same chemistry). Amazingly, these two frits have low boron, they rely on high soda as the flux.
Melt fluidity comparison of frits - 1350F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1350F and held for 15 minutes. Some are still burning off carbon (which seems strange). The two FZ16s are starting to move. Frit 3134 is expanding. 3602 is also starting to melt.
Frit Melt Fluidity Comparison - 1800F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1800F and held for 15 minutes (I already did firings from 1300F-1750F in 50 degree increments, all of them are visible in the parent project). Frit 3110, 3134, 3195, F75 have run all the way down. All of the frits have softened and melted slowly over a range of temperatures (hundreds of degrees). By contrast, Gerstley Borate, the only raw material here, suddenly melted and flowed right over the cliff (between 1600 and1650)! But not before Frit 3602 and FZ16 had done so earlier. Frit 3249 is just starting to soften but F69 (the Fusion Frits equivalent) is a little ahead of it. LA300 and Frit 3124 are starting also. F524, F38, F15 will all be over the end by the next firing. The melt surface tension is evident by the way in which the melts spread out or hold together.
Frits vs. raw materials in glazes: It is not just about the chemistry

This picture has its own page with more detail, click here to see it.
The difference between sourcing fluxing oxides from frits vs. raw materials is significant to say the least. The oxides MgO and CaO normally come from natural mineral that melt high. But common frits that source them soften low. The chemistry in the two cone 6 glazes (compared on this melt flow tester) are the same. But G2934Y4 sources the KNaO from Ferro Frit 3110 instead of feldspar and alot of the MgO from Ferro Frit 3249 instead of talc. Even though Y4 has 10% calcined alumina it still flows much better! Alumina, as a source of Al2O3, is a super refractory material (compared to kaolin, the normal source), the glaze should flow less - but the frits overcome even that to create this amazing fluidity in a matte glaze. The lesson: All glazes have a chemistry, but that cannot be taken in isolation from the materials that source it. Glaze chemistry is a relative, not absolute science.
Melt fluidity comparison of frits - 1400F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1400F and held for 15 minutes. Frit 3134 is still expanding. 3602 is also starting to flow. A number of them are shrinking and densifying like a porcelain would.
Melt fluidity comparison of frits - 1450F
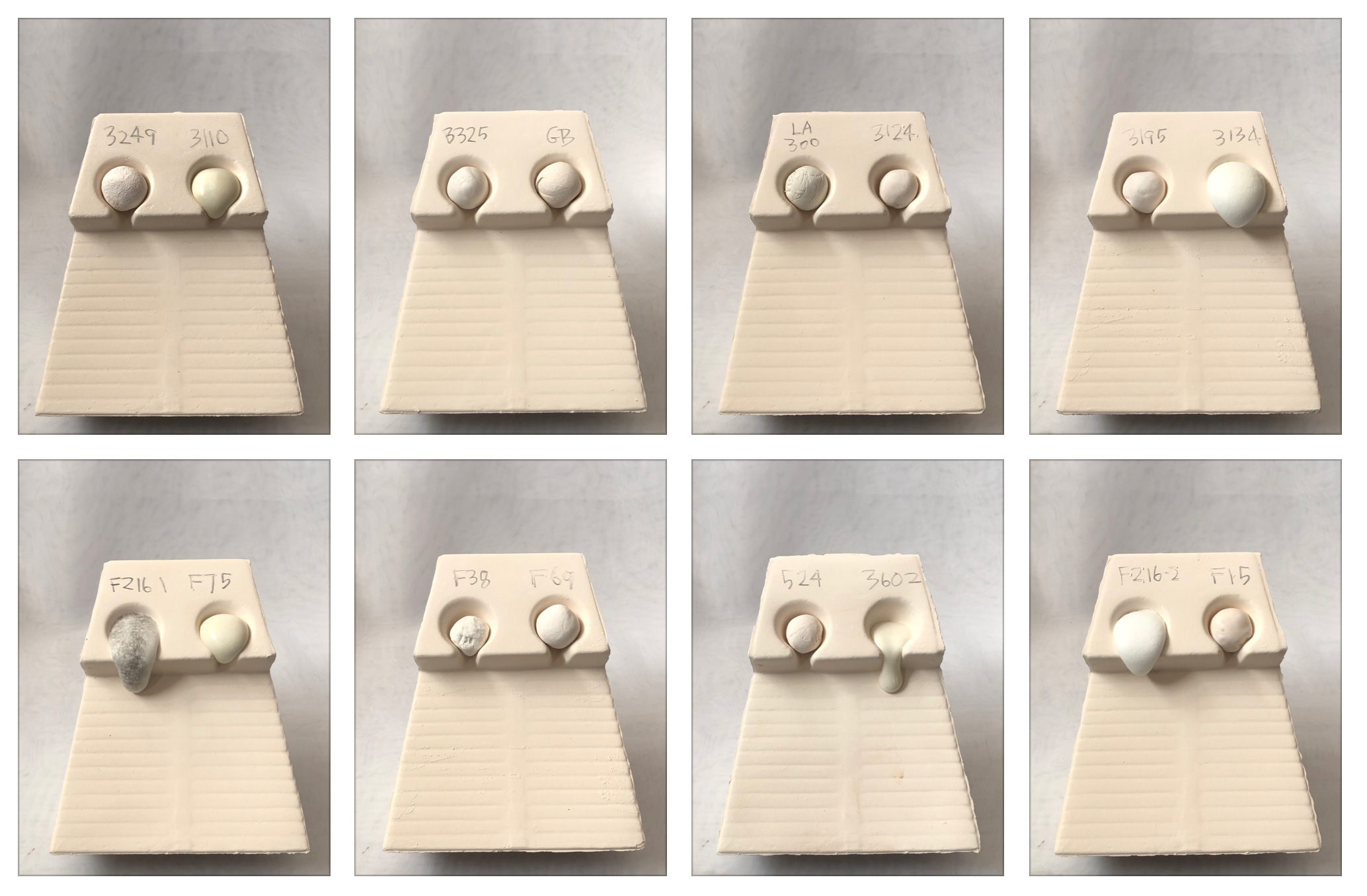
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1450F and held for 15 minutes. Frit 3134 is still expanding. 3602 is blasting out of the gate, taking the lead. F75 is starting to flow.
Melt fluidity comparison of frits - 1500F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1500F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are really starting to move. 3195, F38 and F15 are softening.
Melt fluidity comparison of frits - 1550F
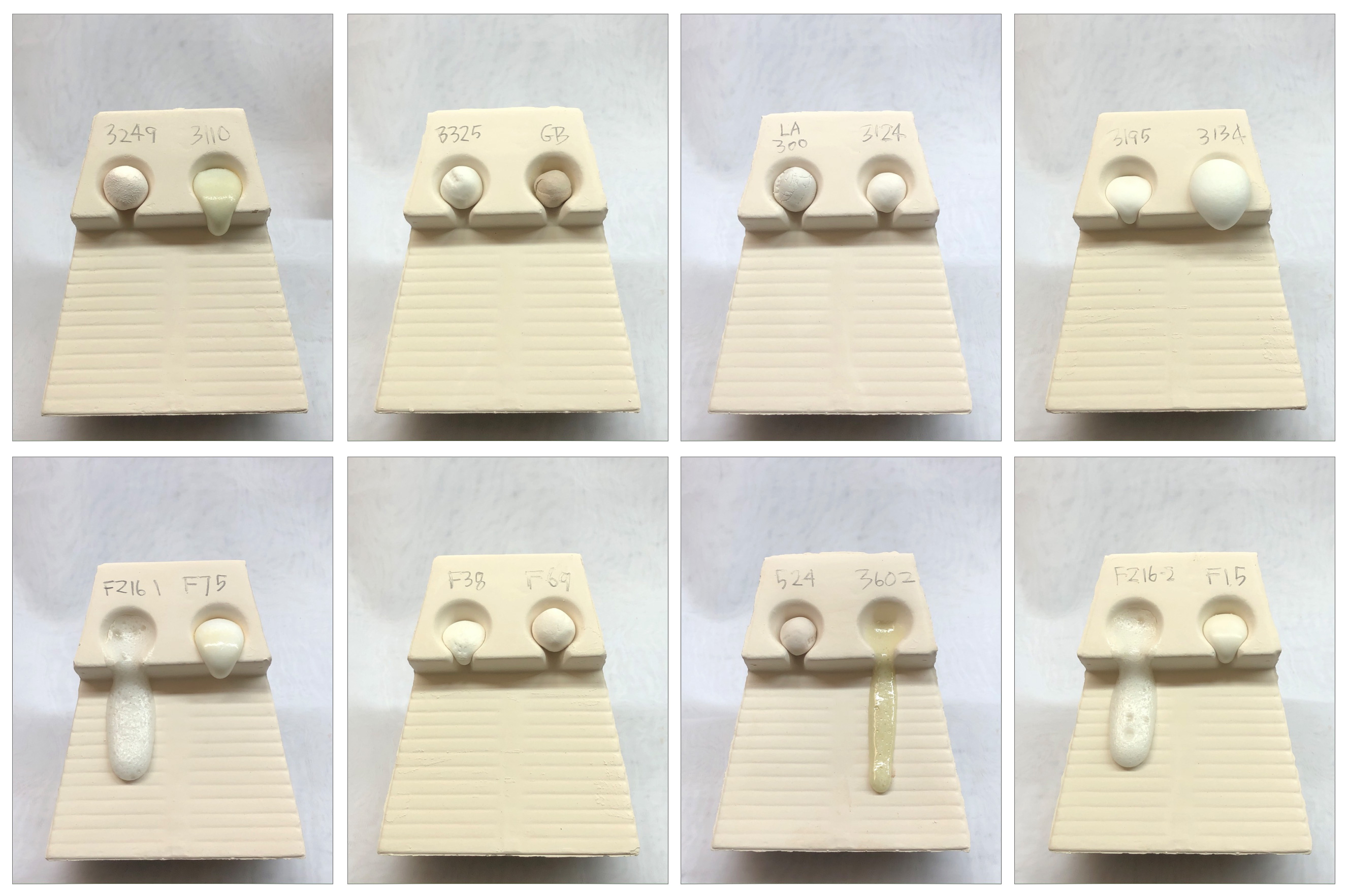
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1550F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are going to be off-ramp by next firing.
Melt fluidity comparison of frits - 1650F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1650F and held for 15 minutes. FZ16 has turned crystal clear and spread out across the runway (has low surface tension). Frit 3110 has so much surface tension that the flow can be lifted off the tester. Since 1600F Gerstley Borate has gone from unmelted to passing all the rest!
Melt fluidity comparison of frits - 1700F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1700F and held for 15 minutes. 3110 is finally starting to move. 3134 also (being full of bubbles). Gerstley Borate has turned almost transparent (because the Colemanite portion of it is now melting). 3195 is looking very well behaved compared to most others, forming a bubble free glass of high surface tension (F15 and F524 are starting to do the same).
Frit Melt Fluidity Comparison - 1850F

This picture has its own page with more detail, click here to see it.
These melt flow tests were fired at 350F/hr to 1850F and held for 15 minutes (I did firings at 50-degree increments across a wide range). It is amazing how active some frits are, even well below normal bisque temperatures! Frit 3110, Frit 3134, Frit 3195, Frit F-75 have all flowed all the way down for many previous temperatures. LA300 and Frit 3124 were just starting at 1800F, look at them now! Frit F-524 and Frit F-38 have gone from half-way at 1800F to water-falling over the end. Frit 3249 is still not out-of-the-gate but Frit F-69 (the Fusion Frits equivalent of 3249) is half-way. Note how the melt surface tension is evident by the way in which the melts spread out or hold together. By contrast, Gerstley Borate (labelled "GB"), the only raw material here, suddenly melted and flowed right over-the-cliff between 1600 and 1650! The best melter of all of them is high-boron high-zinc Frit FZ-16.
Links
| Articles |
Bringing Out the Big Guns in Craze Control: MgO (G1215U)
MgO is the secret weapon of craze control. If your application can tolerate it you can create a cone 6 glaze of very low thermal expansion that is very resistant to crazing. |
| Materials |
Frit
Frits are made by melting mixes of raw materials, quenching the melt in water, grinding the pebbles into a powder. Frits have chemistries raw materials cannot. |
| Materials |
General Frit GF-144
|
| Materials |
Hommel Frit 2GF11C
|
| Materials |
Fusion Frit F-69
A magnesia borosilicate frit having very low thermal expansion and melting point. Commonly used as a substitute for Ferro frit 3249. |
| Typecodes |
Frit
A frit is the powdered form a man-made glass. Frits are premelted, then ground to a glass. They have tightly controlled chemistries, they are available for glazes of all types. |
| Glossary |
Ovenware
Ovenware clay bodies have a low expansion by virtue of materials in their recipe and/or the way they are fired. But potters bend the rules. |
| Glossary |
Abrasion Ceramics
Man-made ceramic surfaces are among the most abrasion resistant materials known. Products made to abrade others are also made from bonded ceramic grains. |
Data
| Co-efficient of Linear Expansion | 4.00 |
|---|---|
| Frit Melting Range (C) | 1900F |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
