| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
A Low Cost Tester of Glaze Melt Fluidity
A One-speed Lab or Studio Slurry Mixer
A Textbook Cone 6 Matte Glaze With Problems
Adjusting Glaze Expansion by Calculation to Solve Shivering
Alberta Slip, 20 Years of Substitution for Albany Slip
An Overview of Ceramic Stains
Are You in Control of Your Production Process?
Are Your Glazes Food Safe or are They Leachable?
Attack on Glass: Corrosion Attack Mechanisms
Ball Milling Glazes, Bodies, Engobes
Binders for Ceramic Bodies
Bringing Out the Big Guns in Craze Control: MgO (G1215U)
Can We Help You Fix a Specific Problem?
Ceramic Glazes Today
Ceramic Material Nomenclature
Ceramic Tile Clay Body Formulation
Changing Our View of Glazes
Chemistry vs. Matrix Blending to Create Glazes from Native Materials
Concentrate on One Good Glaze
Copper Red Glazes
Crazing and Bacteria: Is There a Hazard?
Crazing in Stoneware Glazes: Treating the Causes, Not the Symptoms
Creating a Non-Glaze Ceramic Slip or Engobe
Creating Your Own Budget Glaze
Crystal Glazes: Understanding the Process and Materials
Deflocculants: A Detailed Overview
Demonstrating Glaze Fit Issues to Students
Diagnosing a Casting Problem at a Sanitaryware Plant
Drying Ceramics Without Cracks
Duplicating Albany Slip
Duplicating AP Green Fireclay
Electric Hobby Kilns: What You Need to Know
Fighting the Glaze Dragon
Firing Clay Test Bars
Firing: What Happens to Ceramic Ware in a Firing Kiln
First You See It Then You Don't: Raku Glaze Stability
Fixing a glaze that does not stay in suspension
Formulating a body using clays native to your area
Formulating a Clear Glaze Compatible with Chrome-Tin Stains
Formulating a Porcelain
Formulating Ash and Native-Material Glazes
G1214M Cone 5-7 20x5 glossy transparent glaze
G1214W Cone 6 transparent glaze
G1214Z Cone 6 matte glaze
G1916M Cone 06-04 transparent glaze
Getting the Glaze Color You Want: Working With Stains
Glaze and Body Pigments and Stains in the Ceramic Tile Industry
Glaze Chemistry Basics - Formula, Analysis, Mole%, Unity
Glaze chemistry using a frit of approximate analysis
Glaze Recipes: Formulate and Make Your Own Instead
Glaze Types, Formulation and Application in the Tile Industry
Having Your Glaze Tested for Toxic Metal Release
High Gloss Glazes
Hire Us for a 3D Printing Project
How a Material Chemical Analysis is Done
How desktop INSIGHT Deals With Unity, LOI and Formula Weight
How to Find and Test Your Own Native Clays
I have always done it this way!
Inkjet Decoration of Ceramic Tiles
Is Your Fired Ware Safe?
Leaching Cone 6 Glaze Case Study
Limit Formulas and Target Formulas
Low Budget Testing of Ceramic Glazes
Make Your Own Ball Mill Stand
Making Glaze Testing Cones
Monoporosa or Single Fired Wall Tiles
Organic Matter in Clays: Detailed Overview
Outdoor Weather Resistant Ceramics
Painting Glazes Rather Than Dipping or Spraying
Particle Size Distribution of Ceramic Powders
Porcelain Tile, Vitrified Tile
Rationalizing Conflicting Opinions About Plasticity
Ravenscrag Slip is Born
Recylcing Scrap Clay
Reducing the Firing Temperature of a Glaze From Cone 10 to 6
Setting up a Clay Testing Program in Your Company or Studio
Simple Physical Testing of Clays
Single Fire Glazing
Soluble Salts in Minerals: Detailed Overview
Some Keys to Dealing With Firing Cracks
Stoneware Casting Body Recipes
Substituting Cornwall Stone
Super-Refined Terra Sigillata
The Chemistry, Physics and Manufacturing of Glaze Frits
The Effect of Glaze Fit on Fired Ware Strength
The Four Levels on Which to View Ceramic Glazes
The Majolica Earthenware Process
The Potter's Prayer
The Right Chemistry for a Cone 6 Magnesia Matte
The Trials of Being the Only Technical Person in the Club
The Whining Stops Here: A Realistic Look at Clay Bodies
Those Unlabelled Bags and Buckets
Tiles and Mosaics for Potters
Toxicity of Firebricks Used in Ovens
Trafficking in Glaze Recipes
Understanding Ceramic Materials
Understanding Ceramic Oxides
Understanding Glaze Slurry Properties
Understanding the Deflocculation Process in Slip Casting
Understanding the Terra Cotta Slip Casting Recipes In North America
Understanding Thermal Expansion in Ceramic Glazes
Unwanted Crystallization in a Cone 6 Glaze
Using Dextrin, Glycerine and CMC Gum together
Volcanic Ash
What Determines a Glaze's Firing Temperature?
What is a Mole, Checking Out the Mole
What is the Glaze Dragon?
Where do I start in understanding glazes?
Why Textbook Glazes Are So Difficult
Working with children
Porcelain Tile, Vitrified Tile
Description
A technical overview of the bodies, firing, processes and types of porcelain tiles against the backdrop of the historical development of the process since the 1970s. By Nilo Tozzi
Article
By the title of this article you will note that we use different words to describe tiles with almost zero water absorption (that is without apparent porosity), glazed or unglazed and white or colored by pigment addition.
The production of vitrified tiles has increased a lot during recent years, partially due to world tile production increase, but particularly because equipment and glaze producers have made strong efforts to diversify and improve aesthetic aspects of a product originally born without glaze.
In the Mediterranean area production of vitrified tiles has increased thanks to the new availability of Ukrainian clays particularly suitable to this application. Work is still in progress in order to get an aesthetic improvement and a better process, but the product still shows a promising future.
Right now we can say vitrified floor tiles are replacing normal tiles all over the world. In several countries direct production costs are similar for white bodies but technical characteristics, like abrasion resistance and mechanical strength, are better. The evolution of production processes, specifically press loading and double pressing, has enabled continuous improvements (to the point of becoming suitable as natural stone). The advantages over natural stone are compelling: a consistent industrial production at lower cost.
Description
Porcelain tiles are fully vitrified, glazed or unglazed and can be made using a white or colored ceramic body composed of a mix of clays and feldspars. They are shaped by pressing a powdered body and the fired product has a water absorption less than 0.5% (ISO 10545-3). Specific characteristics are:
- High mechanical strength
- Very good frost resistance
- Can be glazed or unglazed
- Good abrasion resistance.
Unglazed porcelain tiles
The popularity of this type of tile grew rapidly following technology evolution in single firing. We obtained high aesthetic value tiles, mainly polished. Water absorption is 0.1 or less (in order to minimize open porosity after polishing).
Glazed porcelain tiles
Glazed porcelain tiles appeared in the second half of the 1990’s and these contributed to advancement in the overall quality of Italian production. They proved superior to the normal single fired floor tiles (which have higher porosity, lower strength and lower firing temperature). A key practical implication is that the low porosity of porcelain tiles makes them frost resistant. In addition, the higher quality body composition and resultant surface enables the use of a minimum amount of glaze (enough to close surface pores). The high quality surfaces enable a large number of spray and printed color effects (with obvious enhancement of aesthetic value). Water absorption is usually less than 0.5%.
Production
Porcelain tiles have an internal body structure without apparent porosity. The result is a minimum amount of closed pores in a glassy matrix of abundant dispersed crystal phases, this ensures a high mechanical strength. The ratio between glassy matrix and crystal phases also affects the aesthetic properties of tiles (like degree of whiteness and color development).
During firing the viscosity of the liquid phase plays an important role in defining the speed of the sintering process (the rate of crystal phases dissolving and of new phase formation).
Composition
We can obtain specific product characteristics by controlling the glassy matrix to both reduce porosity during fast firing and determine the concentration of residual crystal materials (which reduce fragility of glassy matrix).
Generally porcelain tiles bodies have a 1:1 ratio between plastic components (like clays and kaolin) and non-plastic components (like feldspars and quartz). Sometimes, to enhance glassy phase formation and promote sintering, we add small percentages of calcite, dolomite, talc and wollastonite. The resulting glassy phase has a SiO2 – Al2O3 – M2O composition while crystal phases are predominantly quartz (and a smaller amount of mullite that is formed during firing).
| Italian average oxides composition | |
|---|---|
| SiO2 | 71.0 |
| Al2O3 | 17.5 |
| Fe2O3 | 0.5 |
| TiO2 | 0.5 |
| CaO | 0.8 |
| MgO | 0.5 |
| Na2O | 3.3 |
| K2O | 2.3 |
| L.O.I. | 3.6 |
| Average Shrinkage | 6.0% |
| Average Color | L=67.0 a=2.6 b=11.0 |
Particle size distribution
Once shaping pressure is fixed, as powder fineness increases the number of pores increases while their dimension decreases. Growth of pore diameter takes place during sintering but it it depends largely on pore size before firing. Usually powder residue over 63 microns sieve is maintained at 2.0%.
Pressing
Higher shaping pressure and higher powder moisture decreases the average diameter of pores. The mechanism at play here is this: higher shaping pressure increases the density of green tiles while a higher moisture reduces friction between the particles.
Firing
Firing focuses on developing maximum body density. Soaking temperature is around 1200 ± 20C. We obtain the best mechanical strength when mullite crystals start developing. The strength increases proportionally with the amount of the newly forming mullite phase, until we observe a an opposite effect (because too high a content of crystals yields a body that is too brittle).
During firing of ceramic bodies a solid state reaction evolves and it follows Arrhenius law:
A = Ke -Q/RT
Process is activated by temperature and it starts when the temperature reaches the value of activation which corresponds to energy of activation Q. While at this temperature nothing happens, above it the speed A strongly depends on temperature T (by an exponential factor). K and R are constant factors. From a practical point of view, at activation temperature feldspars start melting and molten glass starts flowing.
Also sintering speed follows Arrhenius law:
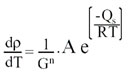
Where: G = particle size - A, n = constant factors - T = temperature - R = gas constant - Q = activation energy of sintering (activation of viscous flow)
From the above equation we can see that the process speed increases with temperature by an exponential factor. Also, when temperature is constant it progressively decreases until it reaches a zero value. The best sintering temperature is that at which the ceramic body quickly vitrifies without bubble formation. As a matter of fact we observe bloating of ceramic bodies when the viscosity of the glassy phase is too low, resulting in bubble growth from residual gases trapped inside material.
Whiteness
Whiteness depends on the selection raw materials and it is inversely proportional to iron and titanium oxides content. When a ceramic body vitrifies attain the lowest degree of whiteness because the glassy phase dissolves iron oxide and becomes colored by it. On the other hand new crystal phases develop enhancing the degree of whiteness. New crystal phases are essentially quartz and mullite, having a diffraction index slightly different from that of the glassy phase, nevertheless these act as opacifying agents (it seems mullite is more efficient than quartz because its particles are smaller). However the degree of whiteness can be improved by adding a crystal phase having a low solubility in the glassy one, like alumina and zirconium silicate.
Colors development
Staining power of added pigments strongly depends on the extent of the glassy matrix and on its composition. Since crystal phases make a composition opaque, a larger glassy phase and less crystals correspond to more color development. Considering the specific composition of bodies, we observe that clays reduce color development because they transform to mullite during firing. Additionally quartz, always present in clays, has a low solubility in the glassy phase. Quartz and mullite act like opacifying materials. Conversely, a high content of feldspars results in more development of color (due to higher amount of glassy phase). It seems there aren’t significant differences of behavior between sodium and potassium feldspars.
HISTORICAL NOTES
In 1860 the English company Mow & Co. started producing mosaics, having a water absorption of less than 3%. These were formed from powder by pressing. Mosaic tiles were also colored by oxides. This can be considered the earliest production precursor of actual porcelain tiles. Later Pilkington and Johnson Tiles also started a similar production process.
Also in 1860, using same English technology, Mosaicos Nello S.A. was founded in Spain, not far from Valencia. It produced 5 cm colored mosaic tiles having low porosity. The venture met with unexpected success, the quality of the product and output of the company was such that we can still see its mosaic tiles in Spain and other countries today. Ceramic body composition was comprised of sandy clays from north-west Valencia, often colored by manganese and iron oxides. It had a water absorption of 2-3%. This company produced mosaic tiles till 1960.
Another precursor of todays porcelain tile is the German clinker brick. Initially they were made by firing red clays to the point of disappearance of apparent porosity. However, practically, water absorption was often higher than 2%. The products were first developed in Holland in the second half of 800’s but production spread to Germany. Tiles made in this way were used for heavy traffic or when frost resistance was necessary. After 1930 in Germany, using the same body composition, tiles were extruded. The tiles were fired without glaze and were made in large sizes and had a water absorption of 1-3%.
After the second world war companies in Italy started imitating German production, but with 10 cm tiles as a maximum. They were quite thin and used locally available red clays. These tiles were used for industrial applications and for heavy traffic floors. Production stopped in the beginning of 1970’s. At the end of the 70’s Italian companies began the production of porcelain tiles using white bodies. This was accompanied by the innovation of single firing (from double firing), the installation of hydraulic press and roller kiln equipment, improvement in aesthetics and technical aspects and by polishing processes. At that time there were 5 companies producing porcelain tiles. Ten years later the first Spanish production lines started and from there the production of porcelain tiles spread to several other countries.
Related Information
Links
| Articles |
Glaze Types, Formulation and Application in the Tile Industry
An technical overview of various glaze type used in the tile industry along with consideration of the materials, processes and firing. |
| Articles |
Inkjet Decoration of Ceramic Tiles
Theory and description of various ceramic ink and inkjet printing technologies for ceramic tile, the issues technicians and factories face, inket printer product overview. |
By Nilo Tozzi
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
