| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Ferro Frit 3134
Alternate Names: F3134, Frit 3134-2
Description: Leadless and low alumina high calcia borosilicate frit
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 19.51% | 0.68 | |
| B2O3 | 22.79% | 0.64 | |
| SiO2 | 45.56% | 1.48 | |
| Na2O | 10.14% | 0.32 | |
| Al2O3 | 2.00% | 0.04 | |
| Oxide Weight | 195.57 | ||
| Formula Weight | 195.57 | ||
Notes
March 2023: This frit IS NOT A SUBSTITUTE for Gerstley Borate, their chemistries are totally different. However, when assessing each glaze that employs GB it is often possible to source some or all of the B2O3 from this frit while adjusting the rest of the recipe to compensate for the differences. Please see the Gerstley Borate page for more information.
A USA pottery frit. A shortage in NA early 2021 led many companies to search for alternatives, Fusion Ceramics inherited some with their F-12. During the process of moving production from a facility in Washington, PA to a state-of-the-art factory in Villagran, Mexico they had to work through a variety of issues (such as Covid 19, raw material shortages, transportation issues and production problems), but they were in full production in April, 2021 (and catching up with back orders).
Ferro now calls it Frit 3134-2. This is a popular frit and has been used for many years as a general-purpose melter across all tempreatures. Equivalents are made by many frit companies. Ferro says that it is "intended for use as a lime and borate source in partially fritted glazes, lead bisilicate glazes and low-cost hobby glazes cone 06-10". But from the viewpoint of ceramic chemistry, this frit is a great 'oxide warehouse', it is useful in so many kinds of glazes, we often use it to showcase the value of Frits in formulating and adjusting glazes as (formulas of oxides rather than recipes of materials).
The reason this is billed as useful in partially fritted glazes is because of how valuable it is in supplying B2O3 (raw B2O3 sources have many issues). It gives us lots of boron along with CaO and Na2O (which most glazes need) but no Al2O3 (so it can be supplied from clay to harden and suspend the slurry).
Several factors make this frit's chemistry so attractive:
-It has almost no alumina. That means, as already stated, that Al2O3 can be supplied by clay, giving the glaze better suspension and hardening properties. Conversely, adding Frit 3134 to a recipe (to supply boron for example) does not require reduction of clay content.
-It has high sodium. That means that its presence enables reducing feldspar content which in turn provides even more opportunity to source Al2O3 from kaolin or clay.
-It has high boron. That gives it a lot of bang for buck as a flux, especially in middle temperature.
-It has a very high CaO content. That makes it useful for developing chrome-tin pinks and maroons. CaO-sourcing raw materials do not normally melt at low temperatures but a frit of this chemistry (high soda and boron) does.
The high expansion of this frit is quite useful since it can be used in a frit blend to create low-temperature glazes with adjustable thermal expansion. The high boron means it can tolerate a very high alumina content from other materials, especially clay. For example, 40 Frit 3124, 40 Frit 3134 and 20 Kaolin is expansion-adjustable since the Frit 3134 can be increased at the expense of 3124 if the glaze is shivering and vice versa if it is crazing.
This frit is often used effectively as part of the strategy to substitute for Gerstley Borate in glazes. It is valuable because it contains lots of sodium and calcium while at the same time sourcing the B2O3. This often enables reducing the feldspar content in the glaze, and then replenishing the oxides contributed by both it and the GB with this frit and kaolin (the latter of which acts to suspend and harden the glaze slurry).
Since Frit 3134 contains no Al2O3, it is not a completely stable glass, it can dissolve in glaze slurries over time and precipitate (to turn the water brown). It is often possible to reduce its amount in favor of the more balanced Frit 3124 (where the glaze has significant feldspar). However, if you drive the clay content too low to accommodate the Al2O3-containing Frit 3124 (using glaze chemistry), you may find the extra hassle of poorer application properties and powdering worth enduring some precipitation issues.
Sub: See also: TAM C-14, General 367-A, 4508
Fusion Frit F-12 has proven a good substitute for this.
Related Information
Crystallization of Rutile at cone 6 completely subdued? How?

This picture has its own page with more detail, click here to see it.
These glazes are both 80% Alberta Slip, but the one on the right employs 20% Ferro Frit 3249 accelerate the melting (whereas the left one has 20% Frit 3134). Even though Frit 3249 is higher in boron and should melt better, its high MgO stiffens the glaze melt denying the mobility needed for the crystal growth.
These common Ferro frits have distinct uses in traditional ceramics

This picture has its own page with more detail, click here to see it.
I used Veegum to form 10 gram GBMF test balls and fired them at cone 08 (1700F). Frits melt really well, they do have an LOI like raw materials. These contain boron (B2O3), it is a low expansion super-melter that raw materials don’t have. Frit 3124 (glossy) and 3195 (silky matte) are balanced-chemistry bases (just add 10-15% kaolin for a cone 04 glaze, or more silica+kaolin to go higher). Consider Frit 3110 a man-made low-Al2O3 super feldspar. Its high-sodium makes it high thermal expansion. It works really well in bodies and is great to make glazes that craze. The high-MgO Frit 3249 (made for the abrasives industry) has a very-low expansion, it is great for fixing crazing glazes. Frit 3134 is similar to 3124 but without Al2O3. Use it where the glaze does not need more Al2O3 (e.g. already has enough clay). It is no accident that these are used by potters in North America, they complement each other well (equivalents are made around the world by others). The Gerstley Borate is a natural source of boron (with issues frits do not have).
These two frits have one difference in the chemistry: Al2O3.

This picture has its own page with more detail, click here to see it.
These two boron frits (Ferro 3124 left, 3134 right) have almost the same chemistry. But there is one difference: The one on the right has no Al2O3, the one on the left has 10%. Alumina plays an important role (as an oxide that builds the glass) in stiffening the melt, giving it body and lowering its thermal expansion, you can see that in the way these flow when melting at 1800F. The frit on the right is invaluable where the glaze needs clay to suspend it (because the clay can supply the Al2O3). The frit on the left is better when the glaze already has plenty of clay, so it supplies the Al2O3. Of course, you need to be able to do the chemistry to figure out how to substitute these for each other because it involves changing the silica and kaolin amounts in the recipe also.
Frit melt fluidity comparison - 1300F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1300F and held for 15 minutes. Some are still burning off carbon (which seems strange). There are two early leaders: Ferro frit 3110 and Fusion frit F75 are starting to deform (they have almost the same chemistry). Amazingly, these two frits have low boron, they rely on high soda as the flux.
Melt fluidity comparison of frits - 1350F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1350F and held for 15 minutes. Some are still burning off carbon (which seems strange). The two FZ16s are starting to move. Frit 3134 is expanding. 3602 is also starting to melt.
Melt fluidity comparison of frits - 1400F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1400F and held for 15 minutes. Frit 3134 is still expanding. 3602 is also starting to flow. A number of them are shrinking and densifying like a porcelain would.
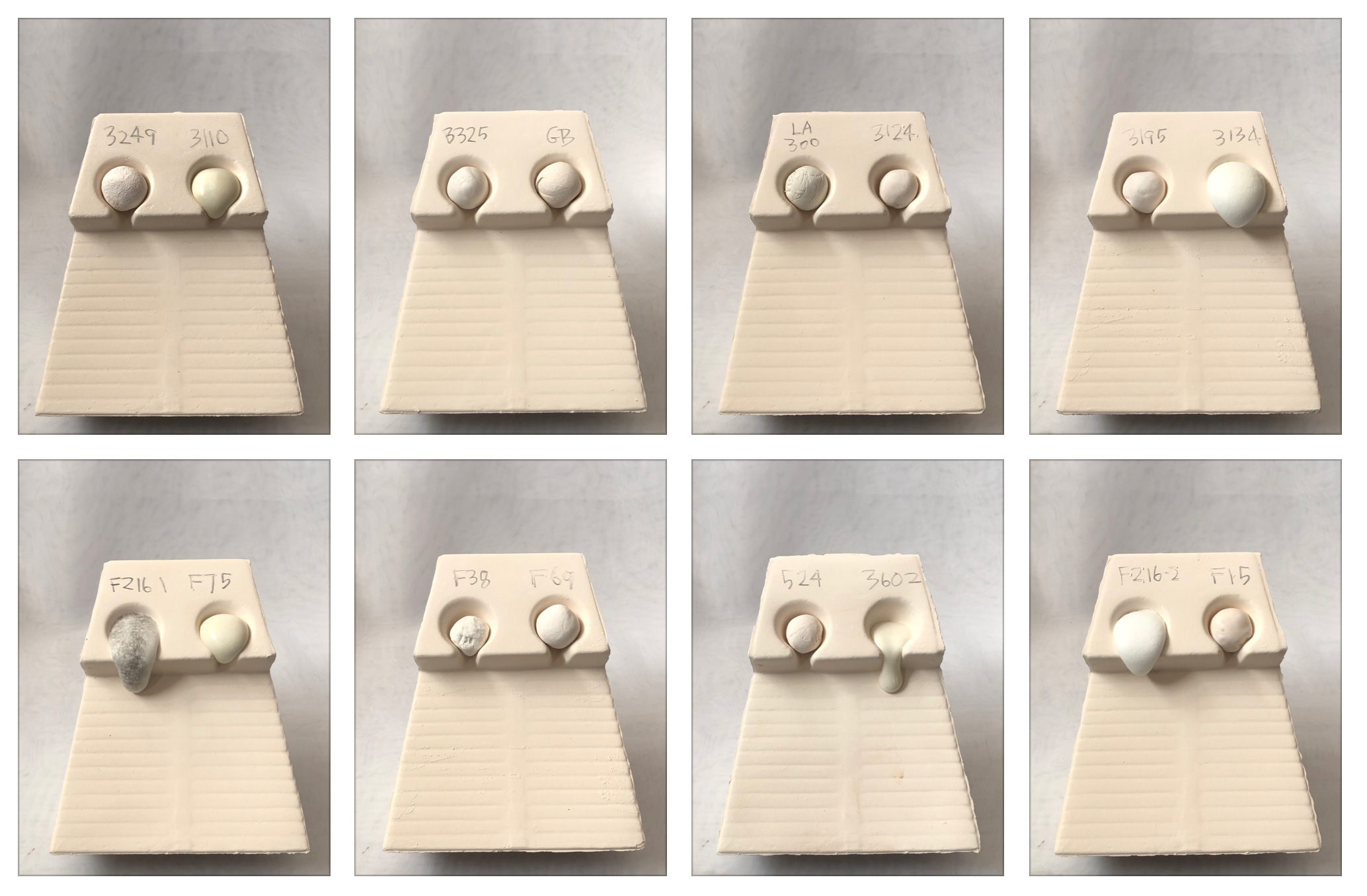
Melt fluidity comparison of frits - 1450F
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1450F and held for 15 minutes. Frit 3134 is still expanding. 3602 is blasting out of the gate, taking the lead. F75 is starting to flow.
Five frits. One kiln at 1850F. Big chemistry drama.

This picture has its own page with more detail, click here to see it.
Five common North American Ferro Frits fired at 1850F on alumina tiles (each started as a 10-gram GBMF test ball and flattened during the firing). At this temperature, the differences are more evident than at 1950F. The degree of melting corresponds mainly, but not only, to the percentage of B2O3 present. Frit 3134 is the runaway leader because it couples that with almost no Al2O3 to stabilize the melt. Notice also the crack pattern, it is also very high in Na2O and thus has a high COE. However, why does Frit 3110 melt so well even though it contains almost no B2O3? Even higher Na2O, it is a powerful flux. Its COE is even higher than Frit 3134, but it is thick enough to have resisted cracking far (but it will in time).
Alberta Slip Rutile-blue needs Frit 3134, it does not work with others

This picture has its own page with more detail, click here to see it.
These two cone 6 mugs have the same glaze recipe: GA6A Alberta Slip base. 4% rutile has been added to each. They were fired in the same kiln using a slow cool schedule. The recipes and chemistry are shown below (the latter gives a clue as to why there is no blue on the right). The mug on the left is the traditional recipe, 80:20 Alberta Slip:Ferro Frit 3134. Frit 3134 melts at a very low temperature and a key reason for that is its near-zero Al2O3 content. Al2O3 in glazes stiffens the melt and imparts durability to the fired glass (normally we want adequate levels in functional glazes). When Al2O3 levels are low and cooling is slower molecules in the stiffening glass have much more freedom to move and orient themselves in the preferred way: crystalline (fast cooling produces a glass). Thus the rutile in the glaze on the left has had its way, dancing as the kiln cooled, producing all sorts of interesting variegated visual effects. The glaze on the right employs Ferro Frit 3195. It has lots of Al2O3 and has contributed enough to stop the rutile dead.
Same glaze, same kiln, same clay: The right one crystallized. Why?

This picture has its own page with more detail, click here to see it.
Well, actually they are not exactly the same. This is 80% Alberta Slip and 20% frit. But the frit on the left is Ferro 3195 and on the right is 3134. By comparing the calculated chemistry for these two we can say that the likely reason for the difference is the Al2O3 content. Frit 3134 has almost none whereas 3195 has 12%. Al2O3 stiffens the glaze melt, that impedes crystal growth. And it stabilizes the melt against running during firing. Frit 3195 is thus much more "like a glaze" than is 3134, it is what Alberta Slip needs to melt as a transparent glass under normally cooling in the kiln.
1700F Frit Melt-Off: Who is the winner? Not the lead bisilicate!

This picture has its own page with more detail, click here to see it.
These were 10g balls melted using our GBMF test. We fired at a temperature far lower than typical bisque, notice how many of them are already melting well! Frit 3602 is lead bisilicate. But it got "smoked" by the Fusion FZ-16 high-zinc, high-boron zero-alumina! Maybe you always thought lead was the best melter. That it produced the most transparent, crystal-clear glass. But that is not what we see here. That being said, notice the lead is not crazing but the FZ-16 is crazing badly, that is a problem for many applications using this frit, it relies on a high percentage of KNaO. Notice something else: Each frit has a distinctive melt fingerprint that makes it recognizable in tests like this. Want to get some of this frit for pottery? You can't, Fusion Ceramics doesn't want to handle retail sales of smaller quantities.
Melt fluidity comparison of frits - 1500F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1500F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are really starting to move. 3195, F38 and F15 are softening.
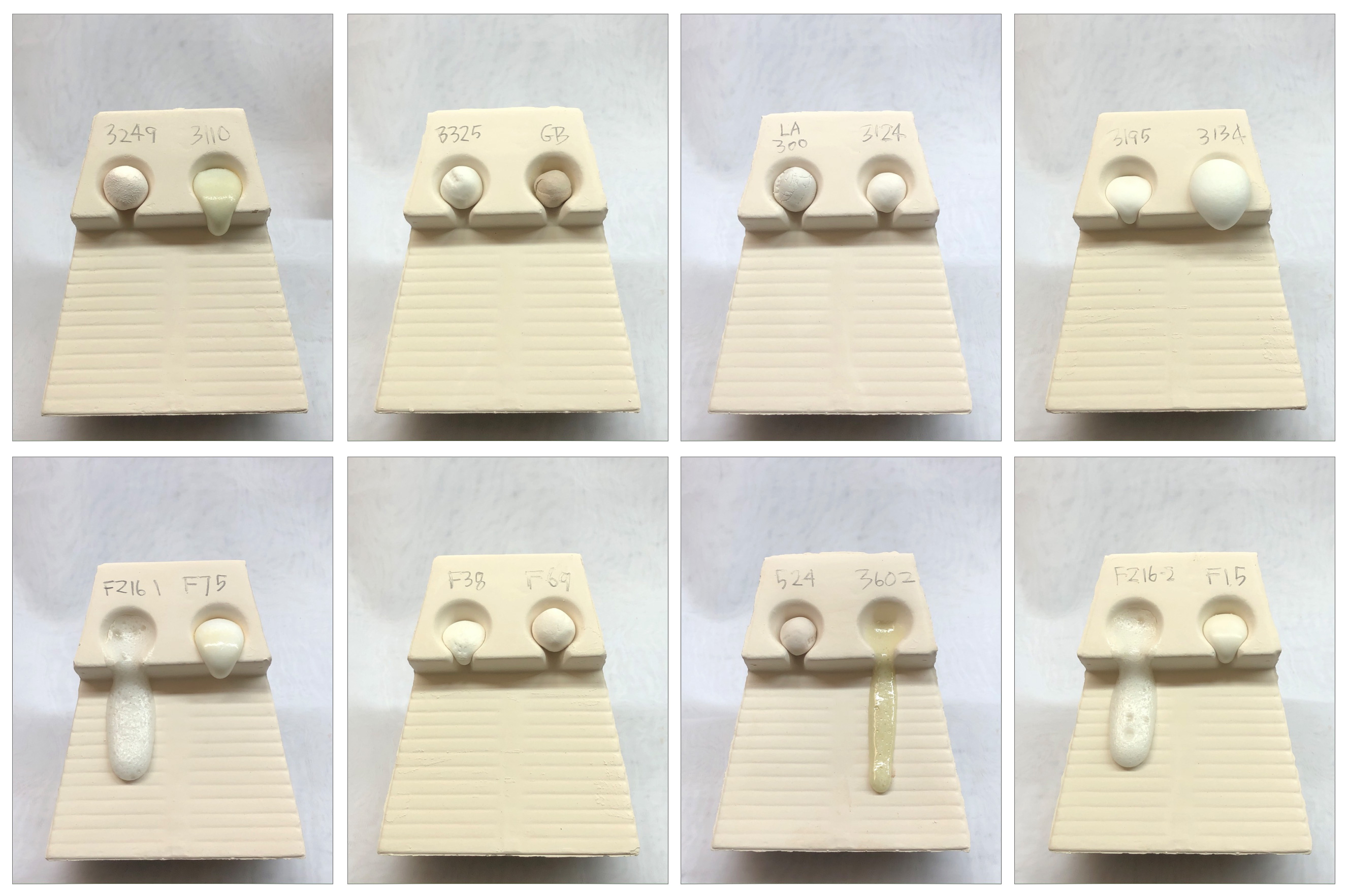
Melt fluidity comparison of frits - 1550F
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1550F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are going to be off-ramp by next firing.
Melt fluidity comparison of frits - 1650F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1650F and held for 15 minutes. FZ16 has turned crystal clear and spread out across the runway (has low surface tension). Frit 3110 has so much surface tension that the flow can be lifted off the tester. Since 1600F Gerstley Borate has gone from unmelted to passing all the rest!
Melt fluidity comparison of frits - 1700F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1700F and held for 15 minutes. 3110 is finally starting to move. 3134 also (being full of bubbles). Gerstley Borate has turned almost transparent (because the Colemanite portion of it is now melting). 3195 is looking very well behaved compared to most others, forming a bubble free glass of high surface tension (F15 and F524 are starting to do the same).
Frit Melt Fluidity Comparison - 1800F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1800F and held for 15 minutes (I already did firings from 1300F-1750F in 50 degree increments, all of them are visible in the parent project). Frit 3110, 3134, 3195, F75 have run all the way down. All of the frits have softened and melted slowly over a range of temperatures (hundreds of degrees). By contrast, Gerstley Borate, the only raw material here, suddenly melted and flowed right over the cliff (between 1600 and1650)! But not before Frit 3602 and FZ16 had done so earlier. Frit 3249 is just starting to soften but F69 (the Fusion Frits equivalent) is a little ahead of it. LA300 and Frit 3124 are starting also. F524, F38, F15 will all be over the end by the next firing. The melt surface tension is evident by the way in which the melts spread out or hold together.
Substitute Ferro Frit 3134, using glaze chemistry, in three glaze types

This picture has its own page with more detail, click here to see it.
Can't get frit 3134 for glaze recipes? Can you replace it with frit 3124? No, 3124 has five times the amount of Al2O3 (the second most important oxide in glazes) and half the amount of B2O3 (the main melter). This ten-minute video presents a glaze chemistry approach that is easier to do than you probably think. It deals with three different glaze recipe types lacking sufficient clay to suspend the slurry. Learn to source the needed oxides from two other Ferro frits, 3110 (or Fusion F-75) and 3195 (Fusion F-2), and end up with at least 15% kaolin in each. A unique approach is required in each situation. Two of the calculations produce improved slurry properties and one yields a recipe of significantly lower cost. If you have a recipe that needs this and need help please contact us.
Fusion F-12 vs Ferro Frit 3134 at cone 04

This picture has its own page with more detail, click here to see it.
On paper, Fusion F-12 has a very similar chemistry to 3134. And in practice it also appears very similar, although a little more melt-fluid than the 3134.
G2926B using Fusion Frit F-12 instead of Ferro 3134

This picture has its own page with more detail, click here to see it.
G2926B is a popular recipe that saw alarm during 2021 because of the difficulty in getting Ferro frit 3134. This motivated us to get a supply of the Fusion equivalent, F-12. This is a demonstration of how effective a melt fluidity test can be in comparing two glazes. Not only does the test compare the degree of melt but also surface reaction to LOI (defects), iron content and melt surface tension.
Frit Melt Fluidity Comparison - 1850F

This picture has its own page with more detail, click here to see it.
These melt flow tests were fired at 350F/hr to 1850F and held for 15 minutes (I did firings at 50-degree increments across a wide range). It is amazing how active some frits are, even well below normal bisque temperatures! Frit 3110, Frit 3134, Frit 3195, Frit F-75 have all flowed all the way down for many previous temperatures. LA300 and Frit 3124 were just starting at 1800F, look at them now! Frit F-524 and Frit F-38 have gone from half-way at 1800F to water-falling over the end. Frit 3249 is still not out-of-the-gate but Frit F-69 (the Fusion Frits equivalent of 3249) is half-way. Note how the melt surface tension is evident by the way in which the melts spread out or hold together. By contrast, Gerstley Borate (labelled "GB"), the only raw material here, suddenly melted and flowed right over-the-cliff between 1600 and 1650! The best melter of all of them is high-boron high-zinc Frit FZ-16.
Two frits with Custer Feldspar

This picture has its own page with more detail, click here to see it.
The GBMF test compares Custer Feldspar with 15% Frit 3110 and 15% Ferro Frit 3134. It is fired at cone 6.
Ferro Frit 3134 is NOT A SUBSTITUTE for Gerstley Borate

This picture has its own page with more detail, click here to see it.
This frit, or any of similar chemistry (e.g. Fusion F-12) IS NOT A 1:1 SUBSTITUTE for Gerstley Borate, their chemistries are too different. That being said, the frit sources lots of B2O3, that makes it a candidate to weave into recipes as the source of boron. To show the difference I have put 100 parts of each in side-by-side recipes in my Insight-live.com account, set the calculation type to non-unity formula and increased the frit until the B2O3 in the two match. Notice it takes 118g of the frit to source the same amount of B2O3 as 100 GB. Notice also that the frit sources almost triple the amount of Na2O per weight unit (that is a big deal because it means the recipe containing the Gerstley Borate to be substituted needs an Na2O-sourcing material that can be reduced to compensate). And the frit sources 3.5 times the SiO2 (other SiO2-sourcing materials in the recipe will need to be cut to compensate). And the Gerstley Borate has significant MgO while the frit has none (so an MgO-sourcing material like talc will be needed). Minor tweaks will also be needed to reduce other sources of CaO (since the frit has quite a bit more). The recipe will also need enough flexibility to do the final matching of Al2O3 and SiO2. The GBMF test confirms the difference at 1700F, these 10-gram balls melted down onto the tiles very differently.
Gerstley Borate vs Frit 3134 melt fluidity comparison

This picture has its own page with more detail, click here to see it.
Here the melt fluidity of Gerstley Borate (GB) is being compared to Ferro Frit 3134 (using a GLFL test). Clearly, these are two very different materials. GB is a clay, Frit 3134 is a man made powdered glass. Notice the GB shrinks to about half its original size by 1600F and then suddenly by 1650 it has exploded out of the starting gate and crossed the finish line! The frit, conversely, slowly softens through the entire 1350-1650 range and then starts down the runway between 1650 and 1700F. While it is clear that frit 3134 is not a direct substitute for the Gerstley Borate (GB) it's more gradual melting make it a better source as a source of B2O3 (boron).
Links
| Materials |
Hommel Frit 14
|
| Materials |
Fusion Frit F-12
|
| Materials |
Pemco Frit P-54
|
| Materials |
Ferro Frit 3124
A commonly available calcium borosilicate frit. |
| Materials |
General Frit GF-111
|
| Materials |
Frit
Frits are made by melting mixes of raw materials, quenching the melt in water, grinding the pebbles into a powder. Frits have chemistries raw materials cannot. |
| Materials |
Ferro Frit 4144
|
| Materials |
Ferro Frit 1077
|
| Materials |
PotteryCrafts Frit P3134
|
| Materials |
Potclays Frit 2273
|
| Materials |
Ceradel Frit 3134
|
| Materials |
Solargil Frit FR8
|
| Articles |
G1916M Cone 06-04 transparent glaze
This is a frit based boron glaze that is easily adjustable in thermal expansion, a good base for color and a starting point to go on to more specialized glazes. |
| Tests |
Density (Specific Gravity)
Measure the density of a dried clay test bar |
| Typecodes |
Frit
A frit is the powdered form a man-made glass. Frits are premelted, then ground to a glass. They have tightly controlled chemistries, they are available for glazes of all types. |
| Typecodes |
Gerstley Borate Substitutes
Many development efforts to create Gerstley Borate substitutes took place during the early 2000s (the initial period when the demise of Gerstley Borate appeared imminent). A number of companies, including Laguna Clays itself, produced and sold these for many years. When Laguna secured another stockpile at the mine and began producing the original material again, interest in substitutes gradually waned. However, the sudden dramatic price increase in 2023 appears to have initiated the process again. Gillespie Borate appears to be the only viable and visible substitute now. Thus, the substitutes listed here are mostly no longer made. Other high-boron materials shown are also no longer available. We continue to recommend sourcing B2O3 from frits instead. Please contact us if you have a specific recipe and we can work with you in your Insight-live account to develop a new recipe that both eliminates the GB and improves overall working and firing properties. |
| Glossary |
Boron Frit
Most ceramic glazes contain B2O3 as the main melter. This oxide is supplied by great variety of frits, thousands of which are available around the world. |
| URLs |
https://digitalfire.com/4sight/datasheets/ferropotteryfrits2008.pdf
Ferro Pottery Frits 2008 |
Data
| Co-efficient of Linear Expansion | 9.47 |
|---|---|
| Frit Melting Range (C) | 1450-1600F |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
