| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Fusion Frit FZ-16
Alternate Names: Fusion Frit FZ16, FZ16
Description: Zero-alumina high-boron zinc flux
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 4.20% | 0.18 | |
| Na2O | 9.40% | 0.36 | |
| ZnO | 15.50% | 0.46 | |
| B2O3 | 30.20% | 1.04 | |
| SiO2 | 40.70% | 1.62 | |
| Oxide Weight | 239.87 | ||
| Formula Weight | 239.87 | ||
Notes
This analysis was confirmed with Fusion Dec 2017. They describe it as "used for textured glazes. Very high in boron and zinc". But that description does not do this justice! No other frit we have ever used, even pure lead bisilicate, melts as well and to the brilliant surface that this does. This is an example of how much sense it makes to use a fritted form of a flux like zinc rather than the pure oxide material (pure zinc oxide cannot produce the brilliant surface that this can). And it is fairly easy to do calculations (e.g. in your insight-live.com account) to substitute zinc in many existing boron-fluxed recipes.
This is an example of a material that should be more commonly available than it is. There are equivalents from other manufacturers, but these likewise, are hard to get for small users. Volume users buy this by the pallet for their production, it's high cost amortizes down well considering the benefit it brings. A typical potter would be aghast at the price.
This is a candidate to replace lead bisilicate for high-gloss glazes. But this frit contains no Al2O3, which means devitrification will likely occur, especially as melt fluidity increases with temperature. The most obvious countermeasure would be adding more kaolin to supply Al2O3 (it will also bring up the melting temperature to better match the lead bisilicate). If the amount of kaolin needed to stop the crystallization brings too much SiO2, then try augmenting with some super-fine ground alumina (e.g. 800 mesh), sufficient temperature and time should dissolve it in the melt. Failing that, feldspar could be used to source the Al2O3 (with an accompanying cut in the frit percentage to keep the KNaO level the same). However, like kaolin, feldspar also brings along plenty of SiO2.
Related Information
Fusion Frit FZ-16 melt flow over many temperatures

This picture has its own page with more detail, click here to see it.
This melt flow tester demonstrates the beautiful crystal-clear glass this zinc frit creates by 1700F. It fits this porcelain without crazing, even though it is very thick and high in sodium (the high zinc and boron are countering it to keep the thermal expansion down). It runs off the end of the runway around 1600F on this GLFL test, rivalling lead bisilicate. This is a more concentrated boron source than even Gerstley Borate. Everything about this material screams “ultra gloss”. What a material to build a fluid-melt reactive super-glaze on!
1700F Frit Melt-Off: Who is the winner? Not the lead bisilicate!

This picture has its own page with more detail, click here to see it.
These were 10g balls melted using our GBMF test. We fired at a temperature far lower than typical bisque, notice how many of them are already melting well! Frit 3602 is lead bisilicate. But it got "smoked" by the Fusion FZ-16 high-zinc, high-boron zero-alumina! Maybe you always thought lead was the best melter. That it produced the most transparent, crystal-clear glass. But that is not what we see here. That being said, notice the lead is not crazing but the FZ-16 is crazing badly, that is a problem for many applications using this frit, it relies on a high percentage of KNaO. Notice something else: Each frit has a distinctive melt fingerprint that makes it recognizable in tests like this. Want to get some of this frit for pottery? You can't, Fusion Ceramics doesn't want to handle retail sales of smaller quantities.
Melt fluidity comparison - 1750F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1750F and held for 15 minutes. Frit 3110 has taken off. And F75, 3195 and 3134 (the latter two having big differences in surface tension).
Frit melt fluidity comparison - 1300F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1300F and held for 15 minutes. Some are still burning off carbon (which seems strange). There are two early leaders: Ferro frit 3110 and Fusion frit F75 are starting to deform (they have almost the same chemistry). Amazingly, these two frits have low boron, they rely on high soda as the flux.
Melt fluidity comparison of frits - 1350F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1350F and held for 15 minutes. Some are still burning off carbon (which seems strange). The two FZ16s are starting to move. Frit 3134 is expanding. 3602 is also starting to melt.
Melt fluidity comparison of frits - 1400F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1400F and held for 15 minutes. Frit 3134 is still expanding. 3602 is also starting to flow. A number of them are shrinking and densifying like a porcelain would.
Alberta Slip fluxed with frit 3195 vs. FZ-16

This picture has its own page with more detail, click here to see it.
Fired at cone 6 using the C6DHSC schedule. On Plainsman M340 and Buffstone. Left: Alberta slip with 20% Ferro frit 3195 (GA6-B). Right: Alberta Slip with 20% Fusion Frit FZ-16 (G3903). This Fusion zinc frit is a super-melter, much better than 3195. A picture cannot do this glaze surface justice! The zinc brings out the red coloration much better. Frit FZ-16 is not readily available, we are hoping companies will eventually stock it. And it produces a more brilliant glassy surface that highlights thickness variations even better. Adding a little extra iron oxide (e.g. 1-2%) would make the effect even richer. One thing to keep in mind: These frits upset the development of rutile or floating blue effects.
Frit Melt Fluidity Comparison - 1800F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1800F and held for 15 minutes (I already did firings from 1300F-1750F in 50 degree increments, all of them are visible in the parent project). Frit 3110, 3134, 3195, F75 have run all the way down. All of the frits have softened and melted slowly over a range of temperatures (hundreds of degrees). By contrast, Gerstley Borate, the only raw material here, suddenly melted and flowed right over the cliff (between 1600 and1650)! But not before Frit 3602 and FZ16 had done so earlier. Frit 3249 is just starting to soften but F69 (the Fusion Frits equivalent) is a little ahead of it. LA300 and Frit 3124 are starting also. F524, F38, F15 will all be over the end by the next firing. The melt surface tension is evident by the way in which the melts spread out or hold together.
Melt fluidity comparison of frits - 1450F
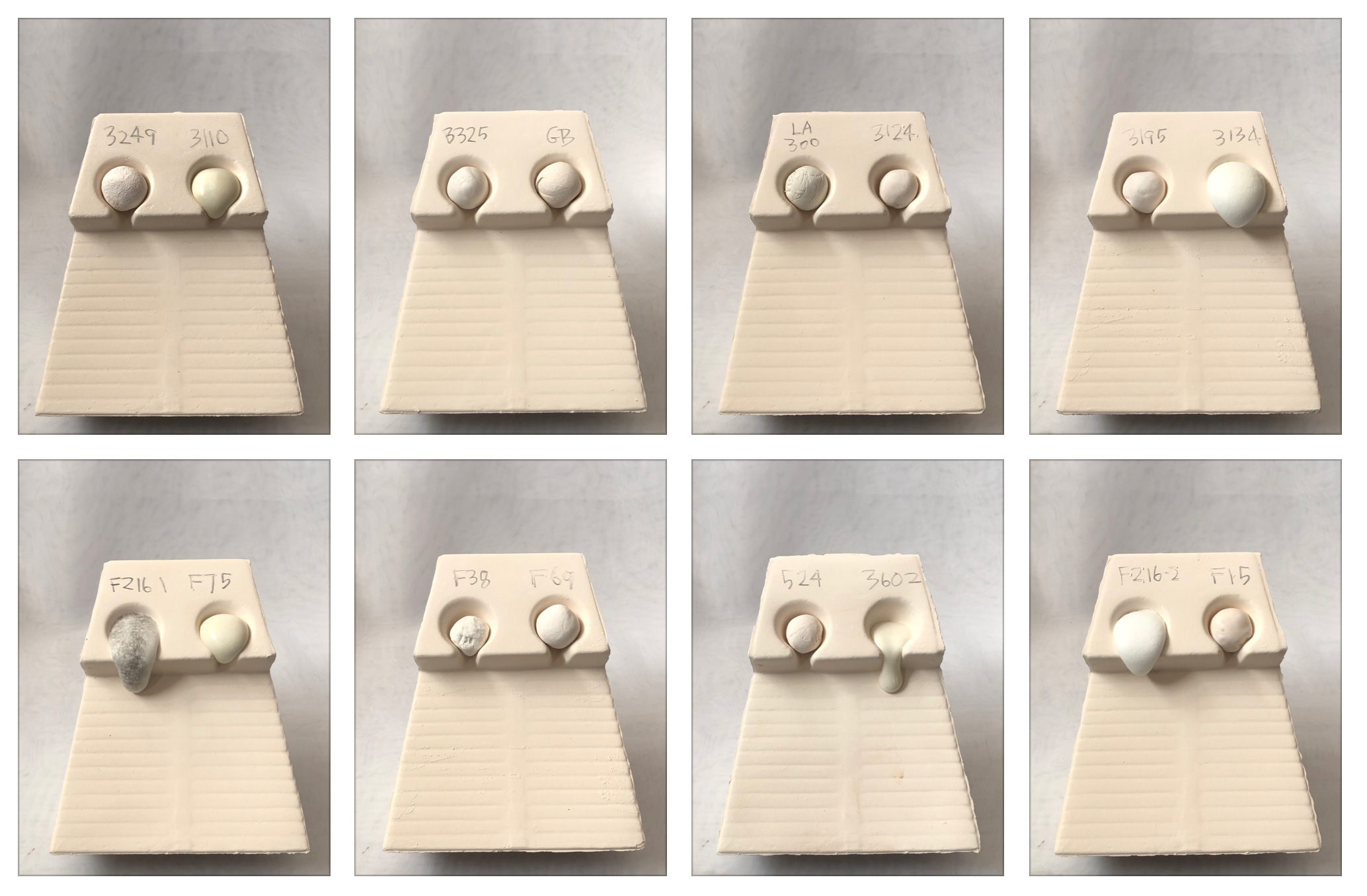
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1450F and held for 15 minutes. Frit 3134 is still expanding. 3602 is blasting out of the gate, taking the lead. F75 is starting to flow.
Melt fluidity comparison of frits - 1500F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1500F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are really starting to move. 3195, F38 and F15 are softening.
Melt fluidity comparison of frits - 1550F
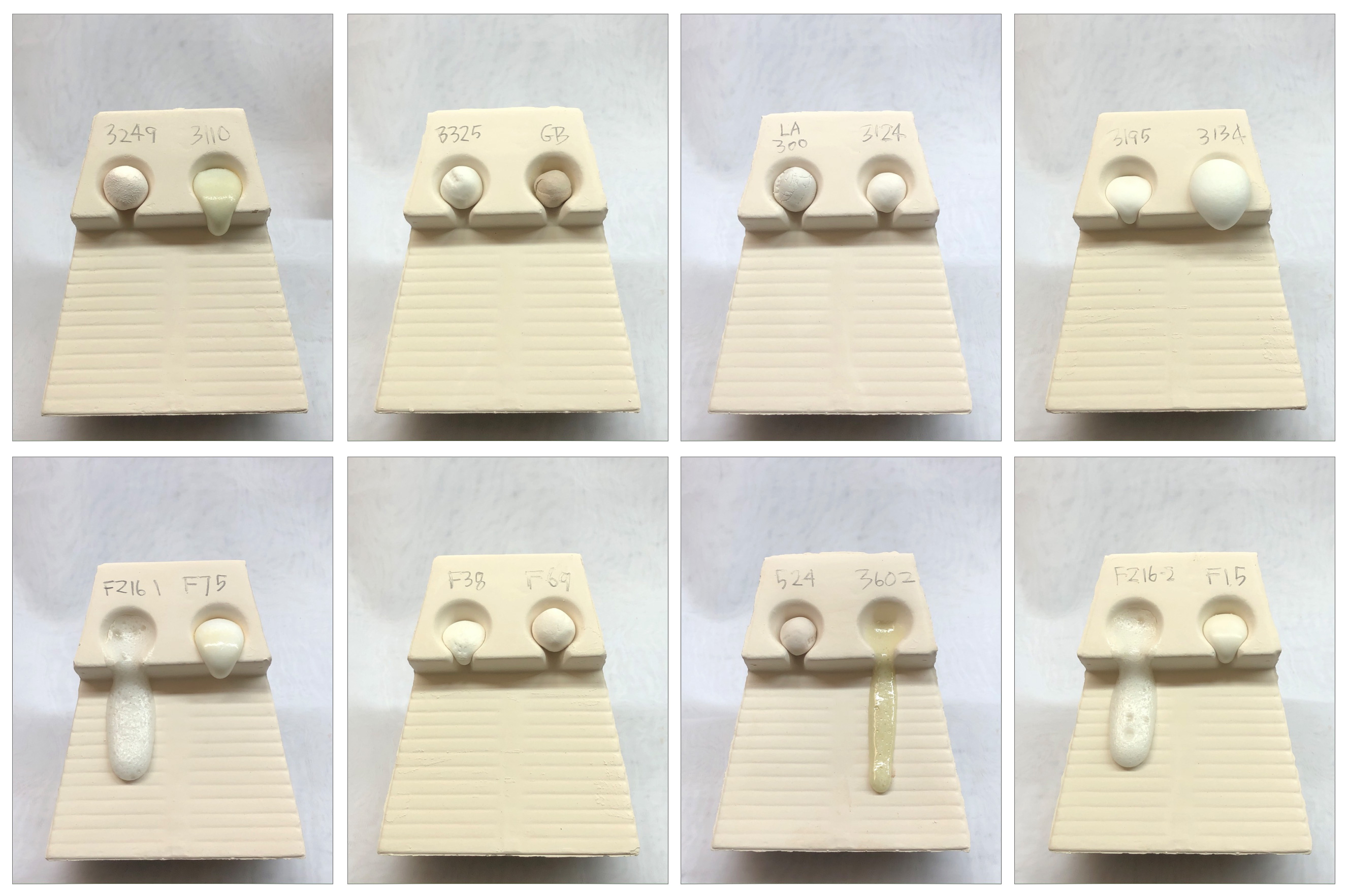
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1550F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are going to be off-ramp by next firing.
Melt fluidity comparison of frits - 1650F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1650F and held for 15 minutes. FZ16 has turned crystal clear and spread out across the runway (has low surface tension). Frit 3110 has so much surface tension that the flow can be lifted off the tester. Since 1600F Gerstley Borate has gone from unmelted to passing all the rest!
Melt fluidity comparison of frits - 1700F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1700F and held for 15 minutes. 3110 is finally starting to move. 3134 also (being full of bubbles). Gerstley Borate has turned almost transparent (because the Colemanite portion of it is now melting). 3195 is looking very well behaved compared to most others, forming a bubble free glass of high surface tension (F15 and F524 are starting to do the same).
Alberta Slip glaze made with boron and zinc frits

This picture has its own page with more detail, click here to see it.
GA6-B (left) is our regular Alberta Slip honey glaze (20:80 Frit:Slip). But the G3903 (mislabelled L3903 here) uses Fusion Frit FZ16, the champion melter of all the frits we have tested. It produces a surface so brilliant it is hard to believe the frit is not leaded. And notice how it has eliminated the bubbling. The G3903 works well down to cone 4.
Frit melt runoff winner

This picture has its own page with more detail, click here to see it.
By 1700F the Fusion zinc frit FZ-16 has already established itself as a better melter than lead bisilicate.
Frit Melt Fluidity Comparison - 1850F

This picture has its own page with more detail, click here to see it.
These melt flow tests were fired at 350F/hr to 1850F and held for 15 minutes (I did firings at 50-degree increments across a wide range). It is amazing how active some frits are, even well below normal bisque temperatures! Frit 3110, Frit 3134, Frit 3195, Frit F-75 have all flowed all the way down for many previous temperatures. LA300 and Frit 3124 were just starting at 1800F, look at them now! Frit F-524 and Frit F-38 have gone from half-way at 1800F to water-falling over the end. Frit 3249 is still not out-of-the-gate but Frit F-69 (the Fusion Frits equivalent of 3249) is half-way. Note how the melt surface tension is evident by the way in which the melts spread out or hold together. By contrast, Gerstley Borate (labelled "GB"), the only raw material here, suddenly melted and flowed right over-the-cliff between 1600 and 1650! The best melter of all of them is high-boron high-zinc Frit FZ-16.
The glossiest slipperiest glaze ever at middle temperature

This picture has its own page with more detail, click here to see it.
This is G3903, a variation on the GA6-B base Alberta Slip honey glaze for cone 6 (but these pieces are fired at cone 5). Its secret is Fusion Frit FZ-16 (high in both B2O3 and ZnO), this is the lowest melting frit we have ever seen. The 20% frit in this recipe is excessive for cone 6, likely 15% or even less would do. Even at cone 4 this glaze is super glossy. An amazing aspect is its slipperiness: Glazed test tiles of this cannot be stacked, they just slide off each other! Put a few coins in that bowl, move it around a little and they slide around and just fly right out! And, I must hold pieces tightly or they will slide out of my hands. Given that this glaze is 80% of a raw native clay and the pieces are made from M340S it is amazing that this small percentage of frit could produce such a brilliant surface. Notice also that it washes away the manganese speckle. Can you get this frit? No. Fusion Ceramics does not sell small quantities - despite many requests that they open a retail sales department for potters.
Is a brilliant metallic glaze surface possible without lead bisilicate?
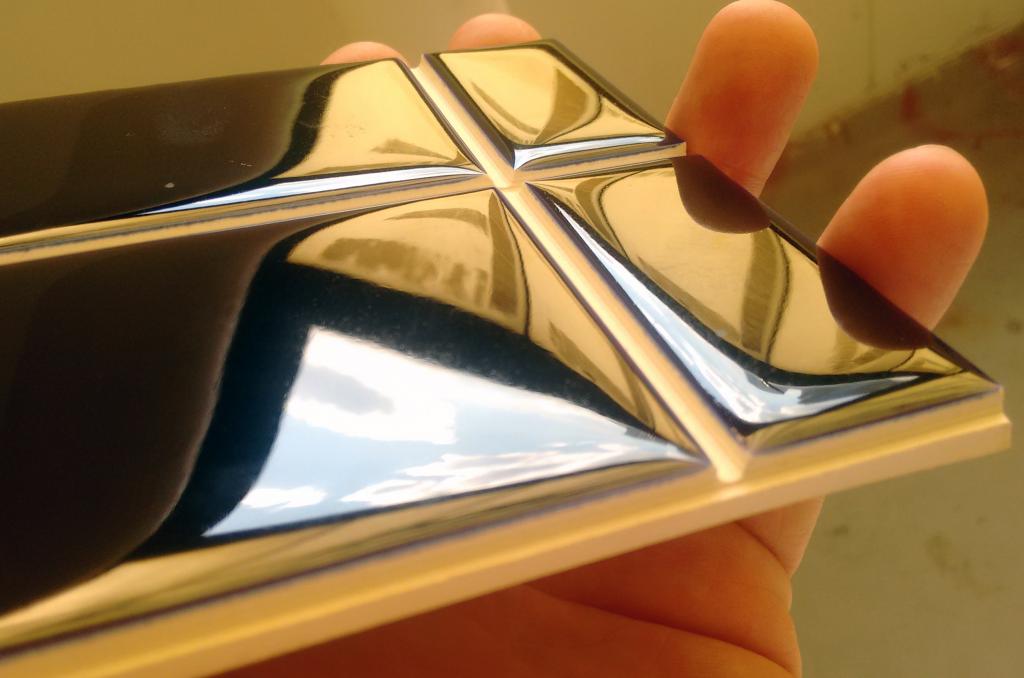
This picture has its own page with more detail, click here to see it.
Lead glazes can have brilliant high gloss surfaces. They can dissolve high percentages of colorants without losing transparency. And do this at unbelievably low temperatures. In Europe the use of lead is still common among potters, in North America it’s use is often met with fear. This picture is courtesy of Cory Lund. He glazes the tiles, cuts the grooves and then fires the tiles again. While working in an area where lead frits are not available Mr. Lund was able to produce a similar surface using lead free Fusion Frit FZ-16, albeit with higher thermal expansion and thus some crazing (but it was not an issue for him). Then, when the frit was not available, he was able to calculate the chemistry and source it using Borax, this worked so well the glaze was even melting too much!
Frits instead of raw zinc, lithium, barium, strontium

This picture has its own page with more detail, click here to see it.
Raw material sources of zinc, lithium, barium, strontium have issues (e.g. precipitates in glaze slurries, toxicity, high drying shrinkage and carbon burnoff that affect laydown and fired surface defects like pinholes, blisters, orange peeling, crystallization). Yet the oxides that these materials supply to the glaze melt - ZnO, Li2O, BaO and SrO, can be sourced from frits which melt much better and remove most of the problems. Consider examples made by Fusion:
-Frit F-493 has 11% Li2O
-F-403 has 35% BaO
-F-581 has 39% SrO
-FZ-16 has 15% ZnO
These frits source other oxides but such are common in most glazes and glaze calculation can be used to retain the overall chemistry. Although these are expensive, the benefits are game changers. But there is a problem: Potters can't get these. Therefore they have difficulty creating the dazzling visual effects of many commercial glazes.
Links
| Materials |
Frit
Frits are made by melting mixes of raw materials, quenching the melt in water, grinding the pebbles into a powder. Frits have chemistries raw materials cannot. |
| Materials |
General Frit GF-110
|
| Materials |
Pemco Frit P-1733
|
| Materials |
Hommel Frit 840
|
| Materials |
Hommel Frit 446
|
| Materials |
Ferro Frit 3824
|
| Typecodes |
Frit
A frit is the powdered form a man-made glass. Frits are premelted, then ground to a glass. They have tightly controlled chemistries, they are available for glazes of all types. |
Data
| Co-efficient of Linear Expansion | 7.90 |
|---|---|
| Frit Softening Point | 1450F |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
