| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Manganese Inorganic Compounds Toxicology
Compounds
Manganese compounds used by potters are inorganic , like manganese dioxide, oxide and manganese carbonate.
Uses
Metallic applications account for most manganese consumption, with about 90% used in steelmaking.
Slags coming from old converters, as in the Thomas process in the steel industry, can be used as fertilizers.
Certain welding operations require the use of electrodes whose coating or alloyed core contains manganese.
The chemical industry uses manganese as a catalyst.
Permaganates are powerful oxidizers.
Manganese has other uses
- As a coloring material
- In the manufacture of dry cells
- In the manufacture of pesticides(Maneb)
- As food additives for livestock
- In the composition of fertilizers
- In pharmaceutical products
- As siccative for paints
- In wood preservatives
- In leather processing
Manganese is an essential mineral for humans and animals. It is necessary for normal bone formation. It has been estimated that a normal 70-kg man has a total of 12mg to 20 mg in his body.
Exposure
The inorganic compounds do not penetrate the body via skin like some organic compounds, such as certain tricarbonyls.
Inhalation of dust or fume is the major route of entry in occupational manganese poisonning. Also inhaled large particles are ingested after mucociliary clearance from the lungs.
Gastrointestinal absorption is generally low (5%). Very few poisonings have occured after ingestion.
Acute intoxication
Metal fume fever
Inhalation of manganse oxide fumes may cause a flu-like syndrome similar to " metal fume fever ", treatment is symptomatic.
Chemical pneumonia
In the case of severe exposure to fumes or dust of various manganese salts, a severe chemical pneumonia may occur.
Acute intoxication by ingestion
Acute intoxication by ingestion rarely occurs and is caused by accidental or voluntary ingestion of a manganese salt (as the ingestion of tablets of potassium permaganate), this chemical causes massive burns of the digestive tract, oedema of the upper respiratory tract and circulatory collapse.
Chronic intoxication
The primary target organ of manganese toxicity is the central nervous system, particularly the extra-pyramidal system ; the lungs may also be injuried in the case of chronic exposure to manganese.
- Central nervous system
Neurological symptoms are caused by inhalation of fumes or dusts of manganese.
The extra-pyramidal system is the main target organ of manganese.
Exposure to heavy concentrations of dusts or fumes for as little as three months may produce the condition, but usually cases develop after 1-3 years of exposure.
In manganese mines where most cases have occurred, the disease has appeared after 10 to 20 years of exposure.
The symptoms may simulate progressive bulbar paralysis, postencephalitic Parkinsonism, multiple sclerosis, amyotrophic lateral sclerosis and progressive lenticular degeneration(Wilson039;s disease).
The best way to diagnose, at an early stage, manganese intoxication is neurological examination.
A standardized questionnaire of neurological symptoms is helpful.
Here are some symptoms, among others, to be looked for in chronic manganese intoxication
- nervousness
- irritability
- memory loss
- tiredness
- insomnia
- muscle weakness
- muscle pain
- trembling fingers
- stiffness of limbs
- difficulty with fine movements
- stuttering
- hoarse voice
- urinary problems
- impotence.
At physical examination your doctor should look for signs of an extra-pyramidal syndrome at its beginning.
- Respiratory tract
Pulmonary tissue does not seem to be the critical target organ of manganese during chronic exposure.
On the other hand, various respiratory symptoms (acute and chronic bronchitis, pneumonia, functional changes of the obstructive type) were observed among workers exposed chronically to manganese at levels higher than those which cause slight neurological disturbances in certain workers.
Reproduction
Manganese could disturb libido according to certain studies. It was demonstrated that a loss of libido and impotence, sometimes preceded by a phase of hypersexuality, was observed among subjects suffering from manganism.
There are contradictory reports as for the effects of manganese on reproduction; however, certain studies showed a reduction in the number of children fathered by male workers during the time when they were exposed to manganese.
In Russia, an excess of spontaneous miscarriages occurred among wives of workers employed in manganese treatment plants.
Teratogenesis
No teratogenic action due to manganese has been described in man.
Mutagenesis and carcinogenesis
The manganese ion has not caused modifications in the DNA among mammals.
In the literature there is no indication suggesting that manganese exerts a cancerogenic action in man. In fact, experimental studies plead rather against such an association.
Exposure
The important thing is your exposure to inorganic manganese, it may vary if you are a pottery factory worker, a teacher, a full-time studio potter or a part-time.
The evaluation of the amount used over a given period of time in clays and glazes is essential in assessing your exposure in a non-parametric way i.e. without the aid of persons specialized in the field of occupational hygiene.
In the wet state, as in moist clays and glazes, these compounds are certainly much less hazardous than as dust.
Pottery factories can financially afford the monitoring of manganese exposure but, it is not the same for artists and craftpersons.
The threshold limit value for dusts of manganese metal and inorganic compounds actually proposed by the ACGIH is 0.2 mg/m3, as total dust.
Prevention
So good house keeping of your studio is very important; to do so you may, among other things, use wet processes, or even a vacuum system whose air is exhausted outside of the workshop.
Avoidance of processes generating unnecessary dust is also importan. To this, we may add work in closed systems and improvement of the general ventilation.
The wearing of an approved dust mask for this kind of hazard is also recommended when the level of exposure seems too high.
Medical surveillance
Often times the only aid to diagnosis is a history of manganese exposure.
- Pre-employment Medical Examination
It aims at searching for the presence of a neurological and/or pulmonary impairment likely to be worsened by exposure to manganese, and it will be used as reference making it possible to better analyze the results of periodical examinations.
Here are some suggested elements
- Complete history taking and physical examination,
- Neurological assessment,
- Spirometry,
- A few psychomotor tests(for instance: evaluation of extremities tremor and reaction (response) time
- Blood and urinary manganese measurements.
- Periodical Examination
Its frequency depends on the severity of exposure and on the legislation in force. It consists in seeking, if possible, with the aid of a well standardized questionnaire, early symptoms of neuropsychological and pulmonary impairment, in repeating the pre-employment examinations and comparing them with the latter in order to detect any risk of excessive impregnation.
It is important to practise this comparison, not only at the individual level but, also at the level of the group of workers.
Urinary manganese levels may be indicative of recent exposures, days to weeks.
Tissue burden and serum concentrations of manganese do not correlate with symptomatology, and symptoms can occurr and progress even after excess metal is cleared from the tissues.
Therefore, serum, whole blood, and urine levels of manganese may be normal in acute or chronic intoxication and cannot be used to predict severity of disease or future progression.
This is contrast with lead encephalopathy.
Among workers, kept away from their job on a temporary basis, and from exposure to manganese dioxide, a good correlation was observed between urinary and blood levels and the index of cumulative exposure, on an individual basis(Lucchini and al.). A correlation was also found between these tests and different neurobehavioral tests.
Tanaka & Lieben however observed a correlation between the urinary excretion and the intensity of exposure, and Japanese authors suggested that manganese excretion higher than 40-50 micrograms/liter corresponds to a level of exposure where lesions can occur (Horiuchi & al.).
- Synopsis of Laboratory Surveillance
Laboratory and diagnostic testing in manganese toxicity
|
Laboratory or diagnostic test |
Abnormal findings |
|
Urinary levels |
>10 mg/L (normal reference, range 0.5 to 9.8 mg/L; up to 50 mg/L for occupational exposure) |
|
Whole blood levels |
>19 mg/L (normal reference, range 8.0-18.7 mg/L) |
| Serum levels | >1.3 mg/L (normal reference, range, 0.3-1.3 mg/L |
| Electroencephalographic parameters |
Low-amplitude, weakened rhythms |
|
Neuropsychiatric testing |
Decreased memory, decreased reaction time, decreased motor coordination |
|
CT scan, magnetic resonance imaging |
Radiodense accumulations of manganese in affected areas, abnormalities in globus pallidus, caudate nucleus, putamen |
| Positron emission tomography | Normal fluorodopa scan |
Treatment
At the beginning of the intoxication, it seems that a chelation treatment with calcium EDTA may favor an improvement of the clinical picture. At an advanced stage of the intoxication this treatment is ineffective. In this case, the treatment is identical to Parkinson039;s disease (levodopa).The usefulness of this treatment is however contoversial.
References
- Occupational Medicine, Carl Zenz, last edition.
- Occupational & Environmental Medicine, Joseph LaDou, last edition.
- Chemical Hazards of the Workplace, Proctor & Hughes, last edition.
- Sax039;s Dangerous Properties of Industrial Materials, Lewis C, last edition.
- Industrial Chemical Exposure, Lauwerys & Hoet, last edition.
- Toxicologie Industrielle et Intoxications Professionnelles, Lauwerys Robert,R.1999
- Encyclopedie Medico-Chirurgicale- Toxicologie-Pathologie Professionnelle - Paris, Lauwerys R, et Roels H., juin 2001.
By Edouard Bastarache
Related Information
A cone 6 black-burning stoneware with a porcelain surface. How?

This picture has its own page with more detail, click here to see it.
Black-burning bodies are popular with many potters. This one is stained by adding 10% raw umber to a buff-burning stoneware. Umbers are powerful natural clay colorants, they have high iron and also contain some manganese oxide. Could a white engobe produce a porcelain-like surface on such a clay body? Yes. L3954B engobe was applied during leather-hard stage to this Plainsman Coffee Clay mug (on the inside and partway down the outside). After bisque, transparent G2926B glaze was applied inside and GA6-B outside. Notice the GA6-B over the engobe fires amber but over the black it produces a deep glossy brown. The engobe was mixed into a thixotropic slurry, as explained on the page at PlainsmanClays.com (see link below), and applied in a relatively thin layer. This porcelain-like result is a testament to the covering power of a true engobe. It is no wonder they are so popular in the ceramic tile industry - a red-burning body can be turned white as a porcelain, which enables all the marvellous glazing and decorating they can do.
Black ash glaze for 20% raw metal pigments: Suitable for functional ware?

This picture has its own page with more detail, click here to see it.
This glaze is 49% Wood Ash, 24% Soda Feldspar and 27% Ball Clay. 10 copper carbonate and 10 manganese dioxide are added to that. This beautiful sculpture was made by Dan Ingersoll, aesthetically this glaze is perfect for it. But there are two red flags here. Significant manganese and copper metal fumes are certain to be generated at cone 10 (they are seriously not healthy) so anyone using this must be very careful. But there is something much more serious - this glaze is being used on functional ware. Copper is well known to destabilize other metals in the fired glass. This 10:10 combination is a perfect storm for leaching heavy metal into food and drink. This is not an argument for the use of commercial glazes, it is one for common sense application of the concept of limit recipes.
Heres evidence that using a black stain is safer than manganese dioxide
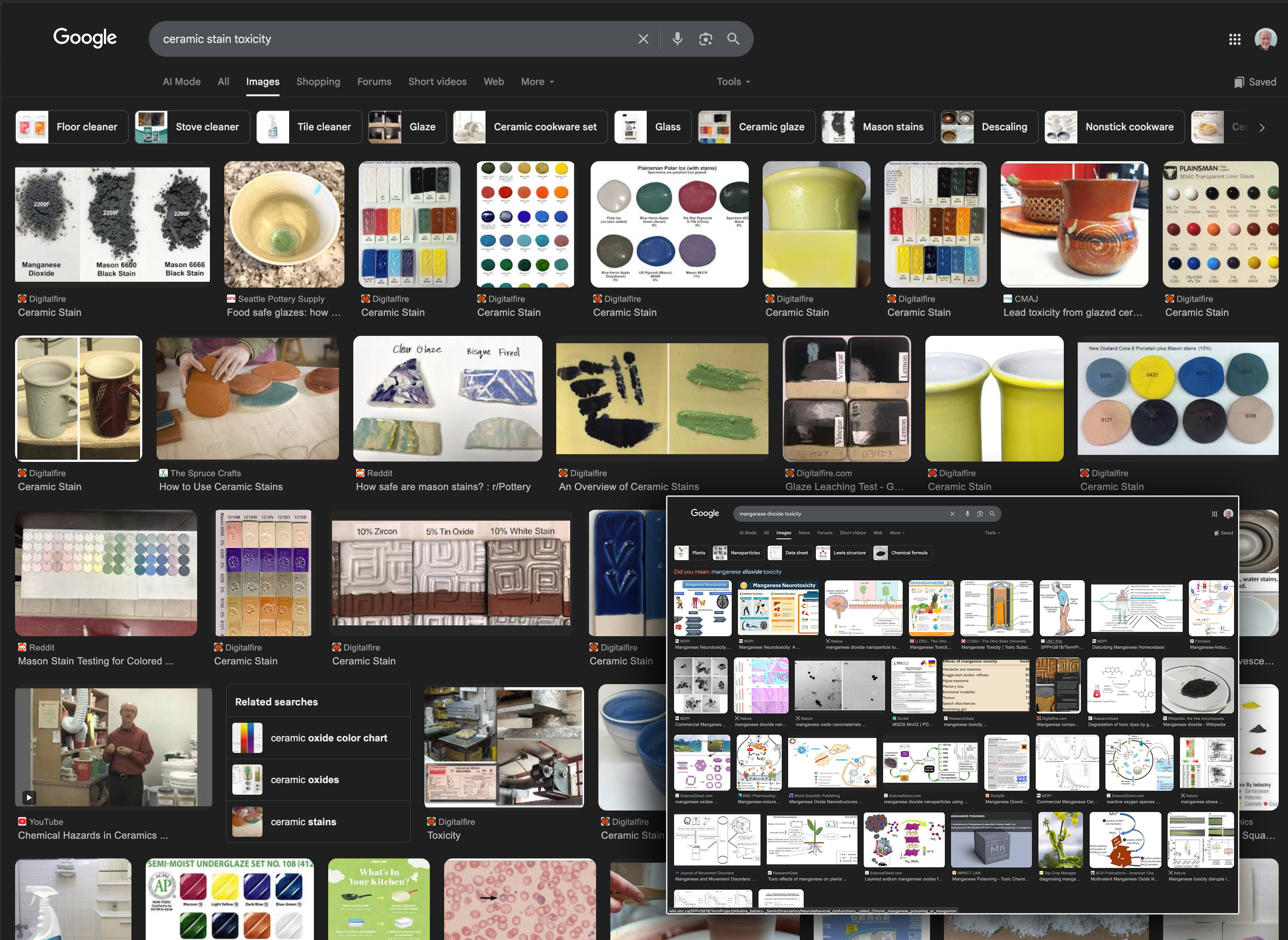
This picture has its own page with more detail, click here to see it.
A search for "ceramic stain toxicity" shows 19 digitalfire hits out of 32. Pretty well all of these pages refer to the increased toxicity of metal oxides over stains. No alarm. However, a search for "manganese dioxide toxicity" (lower right) is alarming (with disturbing words like neurotoxicity, movement disorders, Parkinson’s, distonia, liver disease, iron depletion, etc.). So then, why are so many potters still using recipes for black that contain high percentages of manganese dioxide? Although many black glazes use a combination or iron oxide and cobalt (e.g. 10%/2%), that does not work as well so manganese is still commonly employed (along with other metal oxides). There is a better way: Black stain in a proven base recipe (like GA6-B, G2934, G2926B), as little as 4% is possible. The decreased bioavailability of stains and and the much lower percentage needed make them a no-brainer for coloring glazes more safely.
Links
| Materials |
#2280 Clay
|
| Materials |
A B Abuja Clay
|
| Materials |
Manganese Oxide
|
| Materials |
Manganese Dioxide
A source of MnO used in ceramic glazes and the production of ceramic stains. Commonly made by grinding pyrolusite rock. |
| Materials |
Raw Umber
|
| Typecodes |
Article by Edouard Bastarache
Edouard Bastarache is a well known doctor that has written many articles on the subject of toxicity of ceramic materials and books on technical aspects of ceramics. He writes in both English and French. |
| Hazards |
Manganese in Clay Bodies
Manganese is used to stain clays (using black) and to impart fired speckling (as a decorative effect). It is dangerous? |
| Hazards |
Manganese: Creativity and Illness by Dierdre O'Reilly
A story of one persons struggle with manganese toxicity |
| Hazards |
Manganese Toxicity by Elke Blodgett
A story of the struggle of one person to identify and deal with manganese toxicity |
| Glossary |
Metallic Glazes
Non-functional ceramic glazes having very high percentages of metallic oxides/carbonates (manganese, copper, cobalt, chrome). |
| Temperatures | Manganese compounds may begin to fume (932-) |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
