| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Nepheline Syenite
Alternate Names: Neph Sy
Description: Generic
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 0.70% | 0.06 | |
| K2O | 4.60% | 0.22 | |
| Na2O | 9.80% | 0.71 | |
| Al2O3 | 23.30% | 1.03 | |
| SiO2 | 60.70% | 4.55 | |
| MgO | 0.10% | 0.01 | |
| Fe2O3 | 0.10% | - | |
| LOI | 0.70% | n/a | |
| Oxide Weight | 447.59 | ||
| Formula Weight | 450.74 | ||
Notes
Nepheline Syenite is an anhydrous sodium potassium alumino silicate. Although feldspar-like in its chemistry, mineralogically it is an igneous rock combination of nepheline, microcline, albite and minor minerals like mica, hornblende and magnetite. The Canadian manufacturer describes it as “a naturally occurring, silica deficient sodium-potassium alumina silicate.” It is found in Canada, India, Norway and USSR. We have provided representative chemistry of the Canadian material. This material has a big advantage over feldspar: It does not contain quartz.
Nepheline Syenite has been a standard in the ceramic industry for many years, and is very popular for the whiteness it imparts to clay bodies. Nepheline syenite melts lower than feldspars. For example, it is possible to make a very white vitreous medium temperature porcelain (firing as low as cone 4, but more practically at cone 6). Up to 50% nepheline syenite will be needed at cone 4, 25-35% at cone 6.
Like feldspar, nepheline syenite is used as a flux in tile, sanitary ware, porcelain, vitreous and semi-vitreous bodies. It contributes high alumina without associated free silica in its raw form and fluxes to form silicates with free silica in bodies without contributing free silica itself. This stabilizes the expansion curve of the fired body. It is an excellent tile filler and melter, especially for fast firing. Nepheline syenite is valuable in glass batches to achieve the lowest melting temperature while acting as a source of alumina.
Like talc, this material can also be used at low temperatures to increase the thermal expansion of bodies to make glazes fit better (up to 50% talc is used for this purpose, but it cuts plasticity drastically unless vacuum pugged). Nepheline Syenite does not do this, and potentially less is needed. And it fires whiter.
Since nepheline syenite can be slightly soluble, in pugged bodies it can be responsible for stiffness changes during aging (although admittedly many other factors can also contribute to this). It can more challenging to maintain stable deflocculated slurry bodies using nepheline syenite than with feldspars. However, the place where you may note the solubility of nepheline the most is in glaze slurries containing significant percentages, they can gel over time and the addition of more water to thin the slurry can wreak havoc with application performance (try adding a few drops of deflocculant instead).
Because of its sodium content, high nepheline syenite glazes tend to craze (because of the high thermal expansion of Na2O). Also, since nepheline syenite has more alumina than most feldspars, substituting it into recipes means that on one hand a lower melting temperature is achieved while on the other a more viscous melt results because of the extra alumina.
The picture of the flow test here shows that nepheline syenite by itself is barely beginning to flow and melt at cone 9. However when combined with other materials it will promote melting to a much greater degree than is suggested by its performance alone. Notice that the 400 and 270 mesh particle size versions do not melt differently at this temperature.
Comparison between Canada, Norway and theoretical materials:
SiO2 60.0 56.0 41.1
Al2O3 23.2 24.2 34.9
Fe2O3 0.10 0.11
CaO 0.25 1.2
Na2O 10.8 7.8 15.9
K2O 5.1 9.1 8.1
LOI 0.5 1.5
Related Information
Which is the better flux? Cornwall stone or nepheline syenite?

This picture has its own page with more detail, click here to see it.
Left: Cornwall plus 10% Ferro Frit 3134. Right: Nepheline Syenite plus 10% of the same frit. These are fired at cone 6.
Which is the champion melter of American/Canadian feldspars?
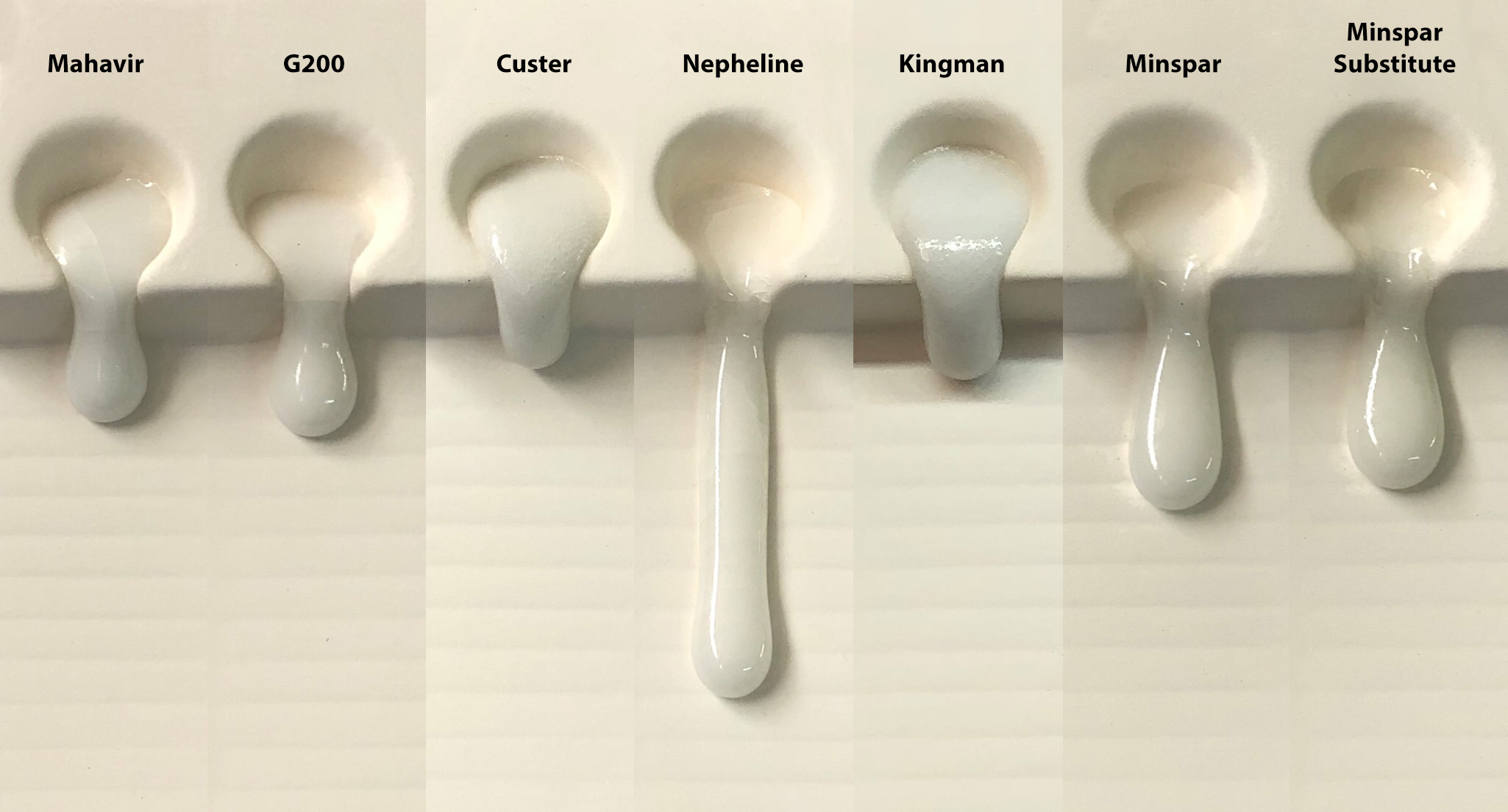
This picture has its own page with more detail, click here to see it.
Feldspars are employed in glaze recipes as melters. So comparing their melt fluidities should be helpful in deciding if one can substitute for another (of course, if possible, a soda-predominant feldspar should be substituted for another soda spar). Feldspars don't melt alone at cone 6 (2200F) so we mixed each with 15% Ferro Frit 3195 (we consider a feldspar a material that sources KNaO). Nepheline Syenite, this is A270, is the champion melter here. Other similar ones can be spotted easily. In the end, degree of melt is a valid consideration in determining if one feldspar is a viable substitute for another in a recipe. Even if the feldspar you want to substitute does not melt as much a little frit can be added to the recipe to make up for the difference (e.g. 3-5%).
The fired color difference between Nepheline Syenite and Custer Feldspar

This picture has its own page with more detail, click here to see it.
These two bars are fired at cone 8 oxidation. The porcelain body recipe is the same (close to equal parts of M23 ball clay, #6 Tile kaolin, feldspar and silica). But the top bar uses Nepheline Syenite (as the feldspar) and the bottom one Custer Feldspar.
Melt flow comparison between Nepheline Syenite 270 and 400 mesh

This picture has its own page with more detail, click here to see it.
This Nepheline Syenite GLFL test for melt flow did not demonstrate much of a difference in melting at cone 9 between 270 and 400 mesh materials.
Cannot get Nepheline Syenite? Here is what to do.

This picture has its own page with more detail, click here to see it.
Nepheline Syenite is similar to a feldspar. I have them open side-by-side here in my Insight-live.com account (the blue panels). In the "Analysis" column notice that Minspar has 9% more SiO2 and 5% less Al2O3. Minspar has 12% fluxes and Nepheline has more than 15%. If a recipe does not contain a significant percentage of Nepheline these differences might be tolerated but what if there is 30% or more? There is no combination of materials that has the chemistry of Nepheline (there is no way to take 10% of the SiO2 out of this feldspar, for example). But it is possible to take SiO2 out of a glaze containing Nepheline Syenite. Notice the two green recipe panels below: The changes made to the one on the right harmonize the oxide chemistry with the original on the left. Those changes were significant: 15% more feldspar, 12% less silica and 2% less kaolin. Notice how easy this was using a little chemistry!
What happens if you blend 35:65 Nepheline:Ball Clay vs Nepheline:Kaolin?

This picture has its own page with more detail, click here to see it.
The piece on the left is 65% ball clay and 35% nepheline syenite. The one on the right is 65% kaolin and 35% nepheline syenite. Both fire vitreous at cone 6. But the glaze is crazing on the kaolin and not on the ball clay. This is because the ball clay contains significant quartz, that raises the thermal expansion and that puts the squeeze on the glaze and prevents the crazing.
Reduction and oxidation porcelains

This picture has its own page with more detail, click here to see it.
Left: Cone 10R (reduction) Plainsman P700 porcelain (made using Grolleg and G200 Feldspar). Right: Plainsman Cone 6 Plainsman Polar Ice porcelain (made using New Zealand kaolin and Nepheline Syenite). Both are zero porosity. The Polar Ice is very translucent, the P700 much less. The blue coloration of the P700 is mostly a product of the suspended micro-bubbles in the feldspar clear glaze (G1947U). The cone 6 glaze is fritted and much more transparent, but it could be stained to match the blue. These are high quality combinations of glaze and body.
Craze city: Feldspar and Nepheline Syenite on cone 10R porcelain bodies

This picture has its own page with more detail, click here to see it.
These were applied to the bisque as a slurry (suspended by gelling with powdered or dissolved Epsom salts). On the left is Custer feldspar, the right is Covia Nepheline Syenite. Notice the crazing (feldspars, and nepheline syenite, always craze because they are high in K2O and Na2O, these oxides have by far the highest thermal expansions).
Covia nepheline syenite bag as of 2021

This picture has its own page with more detail, click here to see it.
Melt fluidity: Cornwall Stone vs. Nepheline Syenite

This picture has its own page with more detail, click here to see it.
Three Cornwall Stone shipments (from 2011 and 2014) fired at cone 8 in melt flow GLFL testers and compared to Nepheline Syenite. Each contains 10% Ferro Frit 3134.
Casey is unloading a truckload of Nepheline Syenite

This picture has its own page with more detail, click here to see it.
Nepheline Syenite is a type of feldspar. We use it in porcelain bodies. The nepheline content determines the temperature at which the body is vitreous. We use this product in preference, where possible over soda or potash feldspar. There are several reasons: It is mined and processed in Canada and is renowned around the world for it's quality. If has a very low iron content, this makes for whiter-burning bodies. It is a more powerful flux than common feldspars. It has been very consistent over many decades.
Covia nepheline syenite demonstrates the incredible fluxing power of Veegum

This picture has its own page with more detail, click here to see it.
The left bars are 5% Veegum and 95% nepheline syenite. By cone 02 (bar #4) it is self-glazing and glass-like with a total shrinkage (plastic to fired) of 15% (less than some porcelains). At cone 03 (bar #5) the porosity is 3% (a stoneware). The right bars are 10% raw bentonite and 90% nepheline (thus the darker color). The top bar (#1) went to cone 3 and has 20% total shrinkage and zero porosity. The bar below it, #3, has 3% porosity. That means that the vitrification process is 6 cones (115°F) ahead in the Veegum-plasticized material compared to the bentonite-plasticized version. Yet the percentage of Veegum is only half as much! This is clear evidence of how powerful of a flux Veegum is.
Custer Feldspar vs Nepheline Syenite at cone 8 oxidation

This picture has its own page with more detail, click here to see it.
Although Nepheline Syenite and Custer Feldspar are used as effective body maturing agents and fluxes in glazes past cone 6, curiously, neither of them melt well by themselves. Thus, both of these come 6 melt fluidity tests add 20% Ferro Frit 3134 to get them flowing. This is a 2021 shipment of the feldspar and a 2022 shipment of the nepheline.
Pure nepheline syenite mug glazed and fired to cone 02

This picture has its own page with more detail, click here to see it.
It is near stoneware strength. How was it possible to make this? Actually, it is 90% nepheline syenite and 10% bentonite. The latter imparts enough plasticity that it can be thrown easily on a potter's wheel. By about cone 1 it begins to warp. This is fired to cone 02 with standard Spectrum low fire glazes. No crazing is evident and the coverage is normal. This really demonstrates the amazing ceramic properties of this material. We aged this for several weeks before throwing and it was stable and unchanged in softness or plasticity.
Intense flashing at cone 10R, courtesy of nepheline syenite tiles

This picture has its own page with more detail, click here to see it.
This flashing method works in reduction or oxidation. It was done by bisque firing a thin nepheline syenite tile leaning against the piece. The tiles are made using a 5:95 mix of Veegum and Nepheline (or a 10:90 mix of raw bentonite and nepheline). Sodium vapours from the tile are deposited on the surface and affect it profoundly enough to influence the glaze firing. A similar effect can be had by spraying on a saltwater solution, it should work whether done prior to the bisque or the glaze firing (of course lots of testing would be required to perfect it).
Links
| Materials |
Soda Feldspar
A feldspar having a KNaO content that predominates in sodium. |
| Materials |
F-4 Feldspar
|
| Materials |
Covia Nepheline Syenite
|
| Materials |
Feldspar
In ceramics, feldspars are used in glazes and clay bodies. They vitrify stonewares and porcelains. They supply KNaO flux to glazes to help them melt. |
| Materials |
Boron Frits
|
| Materials |
Nepheline Syenite Norwegian
|
| URLs |
http://en.wikipedia.org/wiki/Nepheline
Nepheline at Wikipedia |
| Typecodes |
Generic Material
Generic materials are those with no brand name. Normally they are theoretical, the chemistry portrays what a specimen would be if it had no contamination. Generic materials are helpful in educational situations where students need to study material theory (later they graduate to dealing with real world materials). They are also helpful where the chemistry of an actual material is not known. Often the accuracy of calculations is sufficient using generic materials. |
| Typecodes |
Feldspar
The most common source of fluxes for high and medium temperature glazes and bodies. |
| Articles |
Demonstrating Glaze Fit Issues to Students
Glaze and body can both be adjusted to solve crazing and shivering problems. This describes a simple project to create body glaze combinations guaranteed to craze and shiver to demonstrate the principles involved. |
| Oxides | K2O - Potassium Oxide |
| Oxides | Na2O - Sodium Oxide, Soda |
| Oxides | SiO2 - Silicon Dioxide, Silica |
| Minerals |
Nepheline
A feldspathoid silica-undersaturated aluminosilicate mineral. |
| Hazards |
Feldspar
Feldspars are abundant and varied in nature. They contain small amounts of quartz (while nepheline syenite does not). |
Data
| Frit Melting Range (C) | 1100C |
|---|
Mechanisms
| Body Maturity | This material is generally fluxes better than feldspars and produces whiter burning bodies. |
|---|
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
