| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
PbO (Lead Oxide)
Data
| Co-efficient of Linear Expansion | 0.083 |
|---|---|
| Frit Softening Point | 866C (From The Oxide Handbook) |
Notes
-Together with ZnO, PbO is considered one of the Metallic oxide fluxes.
-Reacts easily with silica to form low melting lead silicates of high gloss and deep character. Lead is very easy to use. It is the heaviest oxide and produces incredible colors with most pigments. It is especially recommended for iron tan, browns and reds and titanium yellows and reds. Lead has 'blemish healing' and flow characteristics that are unmatched. Lead glazes tend to have high resistance to chipping. In addition, lead is a 'forgiving material' that tends to hide imperfections on the finished fired surface. Lead glazes have been demanded for fine China for many years, although substitutes have been developed.
-Lead carbonate, a favorite source is highly pure and has a very fine particle size. It also promotes good suspension in raw glazes as well as rapid fusion.
-Lead promotes low expansion, a long firing range, and it decreases viscosity and tendency to devitrify.
-Lead is often used in combination with boric oxide which improves crazing problems and resistance to chemical attack.
-Problems include toxic nature of many forms, volatilization, and loss of gloss during higher firing; dimming of brilliance after long use, and less abrasion resistance. Note that even leaded frits can produce glazes which are soluble if the formulation is faulty or firing is wrong.
Lead Safety
Public and industry attitudes toward lead have shifted in the past few years, and finally most potters and companies are realizing that the narrow parameters within which lead can be used safely (or perceived to be used safely) are just too difficult to work within. Public paranoia is common even though, for example, there are no known cases of lead related illness in the US for domestic manufactured ware.
Inhalation exposure to lead is considered to be significant if the amount of lead in the air is more than half the lead-in-air standard i.e.. >0.075 mg/m3 over an 8 hour working day. Concentration in the human body is significant if it exceeds 40 micrograms/100ml of blood.
The US FDA (Food and Drug Administration) has been a driving force in the move to eliminate lead worldwide. European standards (e.g. European Community Directive 1984) are less stringent and will be modified to allow export to the US. The Compliance Policy Guide adopted by the FDA in 1992 reduces allowable limits dramatically. It is based on the standard 4% acetic acid text as follows:
Category, Samples, Lead Release (micrograms/ml),
Pre 92, Current
--------------------------------------------------
Flatware, 6, 7.0, 3.0
Small Hollowware, 1, 5.0, 2.0
*Cups and Mugs, 1, 5.0, 0.5
Large Hollowware, 1, 2.5, 1.0
*Pitchers, 1, 2.5, 0.5
*New category
The US Consumer Product Safety Commission and the Environmental Protection Agency has also issued voluntary standards.
In the early 90's, industry has been in a compliance mode, adopting quality assurance standards and formulation expertise (i.e. BS 5270, ISO 9000) to meet the challenge and increase credibility. However, environmental concerns and political pressure are forcing the industry to eliminate lead completely or face a total ban. The sectors which most depend on lead are bone china, vitrified hotelware, feldspathic porcelains, and earthenware.
International industry leaders (Corning, Lennox, Mikasa, Noritake, Pfaltzgraff, Royal Doulton, Villeryoy & Boch, Wedgewood) have formed the Coalition for Safe Ceramic Ware to represent the interests of the industry. Only companies with good reputations for producing quality ware are eligible for membership. The CSC called for the reduction of lead limits and was instrumental in the FDA's revisions in Apr 1992. The CSC has continued to develop and apply quality and design control standards which individual members must document and validate with sampling programs. In addition, the CSC has published material to help consumers assure that tableware products are safe to use.
CSC has also taken on the challenge of educating the public as to what it sees as inappropriate. Examples are California's Proposition 65 which requires that consumers be warned if lead levels are more than one-one thousandth of the level at which there is observable effect on human health. For example, a restaurant must warn its patrons with signage. Lead levels of this nature are difficult to measure with test equipment.
Ceramic Oxide Periodic Table

Pretty well all common traditional ceramic base glazes are made from less than a dozen elements (plus oxygen). Go to the full picture of this table and click or tap each of the oxides to learn more (on its page at digitalfire.com). When materials melt, they decompose, sourcing these elements in oxide form. The kiln builds the glaze from them, it does not care what material sources what oxide (assuming, of course, that all materials do melt or dissolve completely into the melt to release those oxides). Each of these oxides contributes specific properties to the glass. So, you can look at a formula and make a good prediction of the properties of the fired glaze. And know what specific oxide to increase or decrease to move a property in a given direction (e.g. melting behavior, hardness, durability, thermal expansion, color, gloss, crystallization). And know about how they interact (affecting each other). This is powerful. A lot of ceramic materials are available, hundreds - that is complicated when individual materials source multiple oxides. Viewing a glaze as a simple unity formula of ceramic oxides is just simpler.
Is a brilliant metallic glaze surface possible without lead bisilicate?
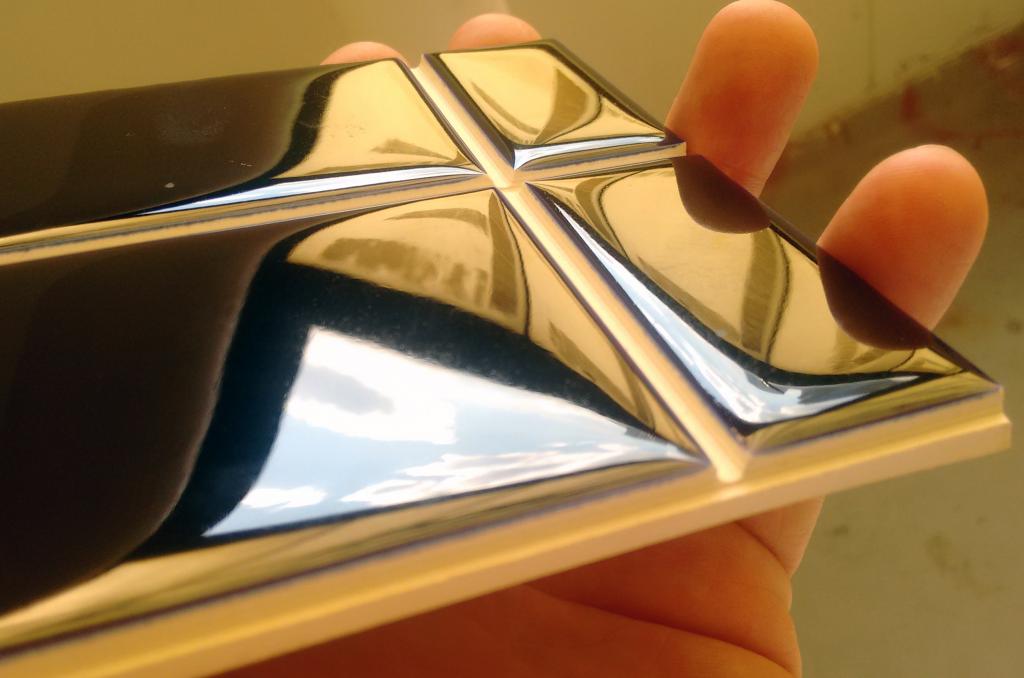
This picture has its own page with more detail, click here to see it.
Lead glazes can have brilliant high gloss surfaces. They can dissolve high percentages of colorants without losing transparency. And do this at unbelievably low temperatures. In Europe the use of lead is still common among potters, in North America it’s use is often met with fear. This picture is courtesy of Cory Lund. He glazes the tiles, cuts the grooves and then fires the tiles again. While working in an area where lead frits are not available Mr. Lund was able to produce a similar surface using lead free Fusion Frit FZ-16, albeit with higher thermal expansion and thus some crazing (but it was not an issue for him). Then, when the frit was not available, he was able to calculate the chemistry and source it using Borax, this worked so well the glaze was even melting too much!
Links
| Materials |
Litharge
|
| Materials |
Red Lead
|
| Materials |
Lead Carbonate
|
| Materials |
Frit
Frits are made by melting mixes of raw materials, quenching the melt in water, grinding the pebbles into a powder. Frits have chemistries raw materials cannot. |
| Oxides | Bi2O3 - Bismuth Oxide |
| Oxides | B2O3 - Boric Oxide |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
