| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Ball Clay
Ball clay is the most common type of secondary clay. They are much more plastic than kaolin because the particle size is much smaller. Ball clays are available with a wide range of plasticities (a product of varying particle sizes). They have higher iron content than kaolins so bodies made from them are not as white. Raw ball clays tend to be grey in color since they commonly contain some lignite. Ball clays are fairly refractory.
Related Information
Carbon burnout in a ball clay

This picture has its own page with more detail, click here to see it.
A broken test bar of ball clay fired to cone 10 reduction. Notice the black carbon core. Ball clays commonly contain carbon, many have a noticeable grey color in the raw state because of this. Notice it has not burned out despite the fact that the clay itself is still fairly porous, the firing was slow and the temperature reached was high. Ball clay typically does not comprise more than 30% of a body recipe so its opportunity to burn away is sufficient. However some specialized bodies have a much higher percentage.
OM4 Ball clay fired from cone 10R (top), 10 down to 4 (downward)

This picture has its own page with more detail, click here to see it.
Ball clays are normally refractory, none of these are vitrified to any extent. The cone 10R bar is yellow because it is stained by the soluble salts present in the material. These are very typical of what most ball clays look like.

This picture has its own page with more detail, click here to see it.
Ball clay and kaolin test bars side-by-side fired from cone 9-11 oxidation and 10 reduction.
High drying shrinkage of Plainsman A2 ball clay (DFAC disk)

This picture has its own page with more detail, click here to see it.
This DFAC test disk shows the incredible drying shrinkage that a ball clay can have. Obviously if too much of this is employed in a body recipe one can expect it to put stress on the body during drying. Nevertheless, the dry strength of this material far exceeds that of a kaolin and when used judiciously it can really improve the working properties of a body giving the added benefit of extra dry strength.
How a kaolin and ball clay compare in a dry performance test
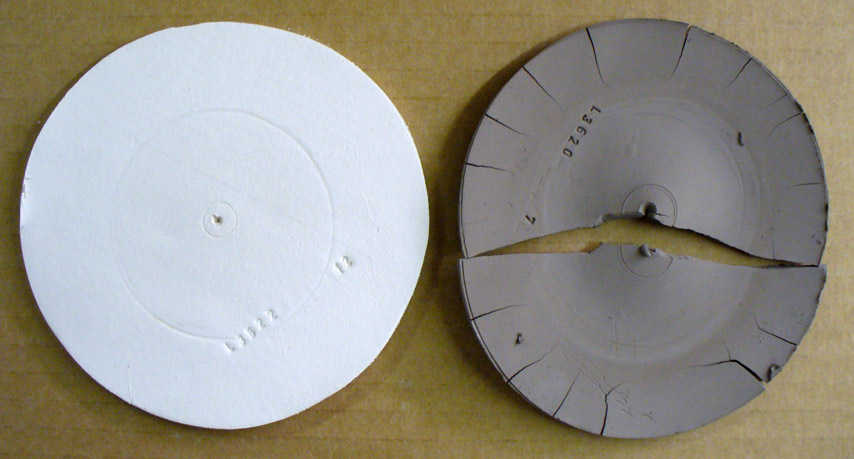
This picture has its own page with more detail, click here to see it.
These are DFAC drying performance disks of a large-particle kaolin (OptiKast) and a ball clay (Plainsman A2). This test reveals a clay's response to uneven drying (these disks are dried with the center portion covered). The kaolin feels smoother yet its ultimate particles are ten to one hundred times bigger than a typical ball clay. Thus it shrinks much less. The ball clay has dramatically lower water permeability, water from the center protected portion resists migration to the outer edge during drying. When the inner section finally dried the outer was already rigid so it split the disk in two and pulled all the edge cracks. Most ball clays shrink more and crack worse than this (cracks concentric to the center also appear). So why use ball clay? This kaolin is so lacking in plasticity it was barely possible to even make this disk. And it is so weak that it can easily break just by handling it. Still, it is useful to make casting bodies. But the ball clay, when used as a percentage of a body mix, can produce highly plastic bodies than can be dried without trouble if done evenly.
Four North American ball clays fired at high temperature

This picture has its own page with more detail, click here to see it.
The top bars went to cone 10R, the others are cone 11 and 10 oxidation (downward from top). They are Gleason, Spinks Blend, OM#4 and NP Blend. It is amazing now similar different ball clays fire in the kiln. Clearly, soluble salts are an issue with all of them (the brownish scum). These bars are much cleaner on the backsides (since the solubles were left on the surface on the fronts during drying). The drying shrinkages, plasticities and fired maturities are also all remarkably similar.
Even heavy soluble salts may not have a significant affect on the glaze

This picture has its own page with more detail, click here to see it.
This is a ball clay. They are known to produce this type of soluble salts when fired at high temperature reduction (the inner salt-free section is such because that part of the tile was covered during drying, so the soluble salts from there had to migrate to the outer exposed edge). If soluble salts fire to a glassy surface they can affect the overlying glaze. But in this case they are not and have a minimal effect.
Particle size drastically affects drying performance

This picture has its own page with more detail, click here to see it.
These DFAC testers compare the drying performance of Plainsman A2 ball clay at 10 mesh (left) and ball milled (right). This test dries a flat disk that has the center section covered to delay its progress in comparison to the outer section (thus setting up stresses). Finer particle sizes greatly increase shrinkage and this increases the number of cracks and the cracking pattern of this specimen. Notice it has also increased the amount of soluble salts that have concentrated between the two zones, more is dissolving because of the increased particle surface area.
Links
| Minerals |
Kaolinite
The most fundamental clay mineral. This mineral is found in nature in its purest form as kaolin. How |
| Glossary |
Secondary Clay
Clays form by the weathering of rock deposits over long periods. Primary clays are found near the site of alteration. Secondary clays are transported by water and laid down in layers. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
