| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Copper Carbonate
Alternate Names: Synthetic Malachite, CuCO3
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CuO | 64.40% | 1.00 | |
| CO2 | 35.62% | n/a | |
| Oxide Weight | 79.54 | ||
| Formula Weight | 123.55 | ||
Notes
Conceptually, copper carbonate is CuCO3, however this form is not normally available in the market (copper carbonate basic is the article of commerce) so the powder should be viewed as a family of compounds.
This material is considered volatile during firing and thus can affect the color of other pieces in the firing. See Copper Carbonate Basic for more information.
Related Information
Original container of World Metal copper carbonate

This picture has its own page with more detail, click here to see it.
Switching copper carbonate for copper oxide in a fluid glaze
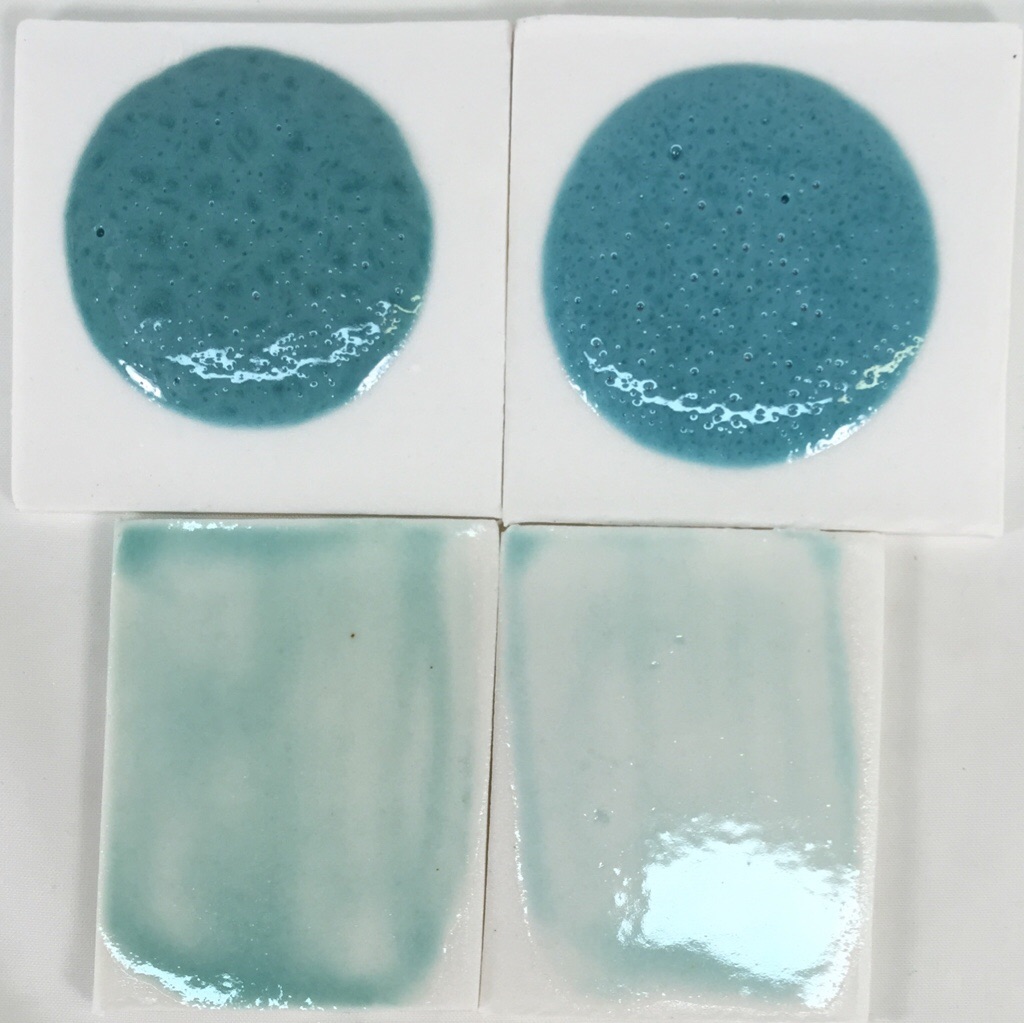
This picture has its own page with more detail, click here to see it.
The top samples are 10 gram GBMF test balls melted down onto porcelain tiles at cone 6 (this is a high melt fluidity glaze). These balls demonstrate melt mobility and susceptibility to bubbling but also color (notice how washed out the color is for thin layers on the bottom two tiles). Both have the same chemistry but recipe 2 has been altered to improve slurry properties.
Left: Original recipe with high feldspar, low clay (poor suspending) using 1.75% copper carbonate.
Right: New recipe with low feldspar, higher clay (good suspending) using 1% copper oxide.
The copper oxide recipe is not bubbling any less even though copper oxide does not gas. The bubbles must be coming from the kaolin.
2% Copper carbonate in two different cone 6 copper-blues

This picture has its own page with more detail, click here to see it.
The top base glaze has just enough melt fluidity to produce a brilliant transparent (without colorant additions). However it does not have enough fluidity to pass the bubbles and heal over from the decomposition of this added copper carbonate! Why is lower glaze passing the bubbles? How can it melt better yet have 65% less boron? How can it not be crazing when the COE calculates to 7.7 (vs. 6.4)? First, it has 40% less Al2O3 and SiO2 (which normally stiffen the melt). Second, it has higher flux content that is more diversified (it adds two new ones: SrO, ZnO). That zinc is a key to why it is melting so well and why it starts melting later (enabling unimpeded gas escape until then). It also benefits from the mixed-oxide-effect, the diversity itself improves the melt. And the crazing? The ZnO obviously pushes the COE down disproportionately to its percentage.
1% and 2% copper carbonate in a cone 6 transparent

This picture has its own page with more detail, click here to see it.
The recipe, G3806B, also contains 2.5% tin oxide. The clear base is the best we found to host the copper blue effect. It was adapted from one found online, we recalculated that to source the Al2O3 more from clay and less from feldspar (to get better slurry properties). Later work was done to try to reduce the thermal expansion of this, but successes in doing that came at a loss in blue color. The COE of this simple enough to fit many bodies without crazing, porcelains having low silica percentages are least likely to fit.
Almost final recipe for cone 6 copper blue - G3806B

This picture has its own page with more detail, click here to see it.
This is the winner of a five-way cone 6 copper blue glaze comparison that started with my dissatisfaction with Panama blue. When I compared these glazes I did not just eyeball them on a tile. I compared the melt flow, thermal expansion and slurry performance of the bases (without the copper and tin). Ball-melt GBMF tests also showed bubble and color development for very thick sections. Then I tried more copper and did more flow tests. I also did leaching tests. Where needed I adjusted recipes to increase clay content (while maintaining chemistry) so the slurries would work better. Without my account at insight-live.com to keep all of this organized it would have been so much more difficult, actually, I probably would not even have bothered with the project. The final recipe, G3806C, was an adjustment to reduce the thermal expansion of this one.
Copper fluxes a matte glaze at cone 6

This picture has its own page with more detail, click here to see it.
4% copper carbonate and 6% rutile have been added to G2934 cone 6 matte base. Using a green stain should prevent this. Or some B2O3 could be substituted with SiO2 (via glaze chemistry).
Why is this cone 6 glaze so different on these two different porcelains?

This picture has its own page with more detail, click here to see it.
Why the difference? The one on the right (Plainsman M370) is made from commodity American kaolins, ball clays, feldspars and bentonite. It looks pretty white-firing until you put it beside the Polar Ice on the left (made from NZ kaolin, VeeGum plasticizer and Nepheline Syenite as the flux). These are extremely low iron content materials. M370 contains low iron compared to a stoneware (less than 0.5%) that iron interacts with this glaze to really bring out the color (although it is a little thicker application that comes nowhere near explaining this huge difference). Many glazes do not look good on super-white porcelains for this reason.
This flow tester indicates copper is not fluxing or bubbling this glaze

This picture has its own page with more detail, click here to see it.
These cone 6 glazes are the same (G3806G), except the one on the right has 3.5% copper carbonate added. Copper is commonly fluxes glazes, making them melt more. But in this case it is not, the clear base is running just as much as the stained one. Either the percentage is not high enough or the host transparent glaze is resistant. Another observation: I was suspicious that the micro-bubbles in the glass matrix were coming from the copper carbonate gassing during firing. But not so, as you can see on this melt fluidity tester, the flow on the left has many more (it appears less melted because of this). In this specific glaze it seems probable that the copper bubbles (generated as it decomposes) act as a fining agent to coagulate and help clear the others.
Pure cobalt carbonate and copper carbonate at 1850F

This picture has its own page with more detail, click here to see it.
Cobalt carbonate (top) and copper carbonate (bottom). Left is the raw powder plastic-formed into a sample (with 2% veegum). Right: fired to 1850F. The CuCO3 is quickly densifying over the past 100 degrees and should begin to melt soon. It is long past the fuming stage.
Copper carbonate fuming

This picture has its own page with more detail, click here to see it.
And example of how copper carbonate fumes during firing. The white sample on the left was near the copper sample, at around 1500F the fumes discolored its facing edge. These are permanent, they do not fire out but get darker with increasing temperature (this is 1950F). The kiln shelf was also discolored outward about half an inch from the copper specimen.
LOI test of common materials flags copper carbonate

This picture has its own page with more detail, click here to see it.
These are pure samples (with 2% binder added) of (top left to bottom right) strontium carbonate, nepheline syenite, cobalt carbonate, manganese dioxide, bentonite (in bowl), 6 Tile kaolin, New Zealand kaolin and copper carbonate. I am firing them at 50F increments from 1500F and weighing to calculate loss on ignition for each. I want to find out at what temperature they are gassing (and potentially bubble-disrupting the glaze they are in or under). Notice how the copper is fuming and spitting black specks on the shelf, this happens right around 1500F (these stains on the shelf darkened considerably when the kiln was fired higher). Yet copper carbonate does not melt and fully decompose until much higher. No wonder it is implicated in cases of glaze blistering and bubbling.
G3806D cone 6 glaze with copper carbonate, copper oxide

This picture has its own page with more detail, click here to see it.
This G3806D fluid melt glaze recipe demonstates the different color characteristics imparted by copper carbonate (left) and copper oxide (at 2%). The carbonate version is bluer and less intense. Copper carbonate is about 65% CuO while the oxide version is 100%. Our supply of the oxide version is not producing any specking (if yours does you may need to sieve or blender mix the slurry).
Links
| Articles |
Leaching Cone 6 Glaze Case Study
An example of how we can use INSIGHT software to determine of a glaze is likely to leach |
| Materials |
Copper Carbonate Basic
This form of copper carbonate is the article of commerce, a mixture of theoretical copper carbonate and copper hydroxide. |
| Materials |
Copper Oxide Black
The purest source of CuO copper oxide pigment used in ceramic glazes. |
| Materials |
Copper Oxide Red
|
| Materials |
Copper Sulfate
|
| Materials |
Copper Hydroxide
|
| Hazards |
Copper Oxide and Carbonate
|
| Hazards |
Copper Compounds Toxicology
|
| Temperatures | Copper Carbonate decomposes to CuO (290-) |
| Typecodes |
Generic Material
Generic materials are those with no brand name. Normally they are theoretical, the chemistry portrays what a specimen would be if it had no contamination. Generic materials are helpful in educational situations where students need to study material theory (later they graduate to dealing with real world materials). They are also helpful where the chemistry of an actual material is not known. Often the accuracy of calculations is sufficient using generic materials. |
| Typecodes |
Colorant
Metallic based materials that impart fired color to glazes and bodies. |
| URLs |
http://en.wikipedia.org/wiki/Copper_Carbonate
Copper Carbonate at Wikipedia |
| Minerals |
Malachite
Copper carbonate mineral |
| Oxides | CuO - Cupric Oxide |
Data
| Frit Softening Point | 500C D |
|---|---|
| Density (Specific Gravity) | 3.70 |
| TGA | See accompanying curve image |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
