| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
PV Clay
Alternate Names: Plastic Vitrox, P.V. Clay, P. V. Clay, Plas Vitrox
Description: White burning plastic feldspar
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 1.00% | 0.14 | |
| K2O | 5.00% | 0.43 | |
| MgO | 0.50% | 0.10 | |
| Na2O | 0.90% | 0.12 | |
| TiO2 | 0.10% | 0.01 | |
| Al2O3 | 12.70% | 1.00 | |
| SiO2 | 76.50% | 10.22 | |
| Fe2O3 | 0.50% | 0.03 | |
| LOI | 2.80% | n/a | |
| Oxide Weight | 780.66 | ||
| Formula Weight | 803.15 | ||
Notes
P. V. Clay is a feldspathic semi-abrasive mineral consisting of clay, mica, feldspar and silica (Moh hardness of 5). It is a product of the hydrothermal alteration of intrusive rhyolite and was mined from a substantial deposit in the Mojave Desert of California. It is not listed on the website of the manufacturer, Protech Minerals, and they no longer support ceramic applications for their materials.
PV Clay had a low loss on ignition (approx 3%), high silica content, good casting properties, low iron content and a long firing range. When fired by itself it begins to vitrify around cone 6 and then progresses to a very glassy white by cone 10 (so bodies employing it can be very white burning). The relatively high potash and soda content give PV Clay a lower PCE than any other commonly available white burning plastic materials. The particle size is significant (the finer the grade, the better vitrification achieved). The material is also plastic (not as plastic as a typical pottery clay, but it is formable). Thus, in a ceramic casting slip or plastic clay body, PV Clay has the unique capability of promoting plasticity from the clay portion, vitrification from the feldspar portion, fired stability and glaze fit from the quartz portion and whiteness from the low iron content.
This made it useful in a very wide range of clay body types, both vitreous and non-vitreous. It was viewed by some as "nature's pure porcelain".
-In artware casting (in high talc recipes) it imparted resistance to glaze crazing (because of its high silica content), resistance to dunting (because the silica is fine grained), white color and good casting properties. The popular "California Artware Body" recipe was 3 parts California Talc, 1 part PV Clay and 2 parts ball clay.
-PV was a practical material for use wall tile bodies (because of the whiteness, plasticity and high silica content).
-It was practical for floor tile bodies (because it vitrified the body).
-It was possible to make a stoneware body of only ball clay and PV (it supplied the feldspar, silica and white burning kaolin) and the ball clay supplied the plasticity.
The flux content, low iron and plasticity of PV Clay made it a practical glaze material also. A simple 50:50 mix of PV Clay and Gerstley Borate was used widely as a transparent glaze at cone 5-7. A mix of 80:20 PV and calcium carbonate produced a cone 10 transparent glaze.
P.V. Clay is also used as a filler in rubber products, as a mild abrasive in polishes and cleansers, and as an extender for coatings, compounds, and other industrial products.
While the manufacturer, Protech Minerals, claimed consistency, they did not change the data sheet over a twenty year period. And customers received shipments having wide variations in fired maturity and plasticity. They claimed the following data (which is helpful in creating blends to duplicate it):
Particle Size Distribution
(typical Sedigraph Method)
40 91.0 (below 40 microns)
30 77.5
20 67.0
15 61.5
10 56.0
7.5 51.0
5 43.0
4 40.0
3 35.0
2 31.0
1 23.5
Mineralogical Analysis:
----------------------
Silica 46%
Feldspar 34%
Kaolinite 24%
Sulphur .03
LOI 2.3%
Specific Gravity 2.65
pH 9.2
Dry Brightness 82
Fired Brightness 80
Apparent Density
(lbs./ft3) 27
Tapped Density 66
Unfired Physical Properties:
Rate of Cast, Thickness 15 min
(specific gravity 1.5) 1.21 cm 1.27 cm 1.83 cm
Percent water of plasticity: 28 29 32
Percent dry shrinkage 4.2 4.4 4.8
Cone 03 Cone 5 Cone 8
---------------------- -------------------- --------------------
LOI 1.2 2.4 2.9 1.6 2.7 3.0 2.2 2.7 3.3
Absorption: 19.5 18.8 20.6 11.7 6.4 .6 6.0 .2 .1
Fire Shr: .5 1.0 1.0 5.2 8.1 10.7 8.0 9.9 10.1
MOR: 1051 1202 1807 3357 4482 6717 4812 6257 6900
Color light buff Brown Gray lt med med
grey gray gray
PCE Cone 18
Melting Point: 2700F
At one time, PV Clay was made by a wet milling process and was known to the trade as "Plastic Vitrox" from whence it derives its name. Some quotations on the chemistry show more alumina and silica than is show here. For example: 1RO 1.69Al2O3 14.64SiO2.
There has been much activity in recent years among clay body manufacturers to develop equivalents. This is quite practical since the natural míneralogy of the material is feldspar, clay and quartz. To work well as a substitute, an equivalent should have plasticity and vitrify to the same degree.
Related Information
The same raw material, same temperature, different batches!
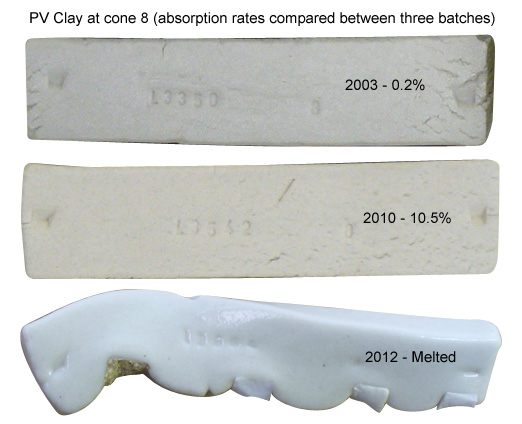
This picture has its own page with more detail, click here to see it.
Test bars of three different batches of PV Clay (a plastic feldspar) fired at cone 8 (porosity is shown). This is how much a manufactured material can vary, obviously body blenders need to be watching the properties of the materials they receive. This raw material is employed in many clay bodies in North America as a plastic flux. The chemistry and physical properties of this material can be duplicated with a blend of kaolin, feldspar and silica.
A shipment of PV Clay at its worst: Not vitreous

This picture has its own page with more detail, click here to see it.
These test bars were fired from cone 10R and 11 oxidation down to 7. The porosity first dips under 1% at between cone 11 and 12 (rather than the normal cone 4)! By cone 8 it rises to 10%. Fired shrinkage is 8.5% at cone 11 dropping to 5% by cone 8. Drying shrinkage is about 3.5%, up to 1.5% less than normal. Normally, PV clay is a naturally occurring mid temperature plastic porcelain. This, by contrast, is a non-plastic, high temperature stoneware (it has 2.5% porosity at cone 10R, so it is far from being a high fire porcelain). Any company that used this shipment in its clay body production faced dramatic changes in their fired and plastic properties if they have been using the more melted version before. The level of maturity presented by these test bars was considered more "normal" by body manufacturers.
Fired bars of PV Clay and a substitute fired at six temperatures

This picture has its own page with more detail, click here to see it.
On the left is a 2013 shipment of PV Clay. These fired test bars are from cone 10R and 5 oxidation down to 1. This is PV theoretically as it used to be, a plastic feldspar. It is melting at cone 10R and reaches almost zero porosity at cone 4 (by cone 3 it has 4.5% and by cone 2 it has 11.5%). Fired shrinkage is 10% by cone 4, dropping to 6% by cone 2. Dry shrinkage is about 5%. The substitute blend on the right, L3894, is 38% silica, 44% Custer feldspar, 12% Pioneer kaolin, 3.5% bentonite and 2.5% dolomite (it calculates to a very similar chemistry). And these fired bars exhibit shrinkage and densities very similar to the PV Clay at every temperature. However, the drying shrinkage is about 1% lower and the fired color is darker (we later changed the recipe to use #6 Tile kaolin to add plasticity and Nepheline Syenite to make it whiter-burning). Of course, one could adjust this recipe further to suit (e.g. reduce its maturity by reducing the feldspar, reduce its plasticity by cutting the bentonite).
Links
| Materials |
FC-1 Filler Clay
|
| Materials |
Plastic Vitrox
|
| Typecodes |
Feldspar
The most common source of fluxes for high and medium temperature glazes and bodies. |
Data
| Pyrometric Cone Equivalent | 17 |
|---|
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
