| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
3D Printing a Clay Cookie Cutter-Stamper
A 3-minute Mug with Plainsman Polar Ice
A Broken Glaze Meets Insight-Live and a Magic Material
Accessing Recipes from "Mid-Fire Glazes" book in Insight-Live
Adjusting the Thixotropy of an Engobe for Pottery
Analysing a Crazing, Cutlery-marking Glaze Using Insight-Live
Compare the Chemistry of Recipes Using Insight-Live
Connecting an External Image to Insight-Live Pictures
Converting G1214M Cone 6 transparent glaze to G1214Z matte
Create a Synthetic Feldspar in Insight-Live
Creating a Cone 6 Oil-Spot Overglaze Effect
Design a Triangular Pottery Plate Block Mold in Fusion 360
Designing a Jigger Mold for a Bowl Using Fusion 360 CAD
Downloading and 3D-Printing a 3MF file
Draw a propeller in Fusion 360 for use on an overhead propeller mixer
Drawing a Mug Handle Mold in Fusion 360
Drawing a Mug Mold Using OnShape CAD
Enter a Recipe Into Insight-live
Entering TestData Into Insight-Live
Fine tune the thixotropy of a glaze or engobe slurry
Getting Frustrated With a 55% Gerstley Borate Glaze
How I Developed the G2926B Cone 6 Transparent Base Glaze
How I Formulated G2934 Cone 6 Silky MgO Matte Glaze Using Insight-Live
How to Apply a White Slip to Terra Cotta Ware
How to Paste a Recipe Into Insight-live
Importing Data into Insight-live
Importing Desktop Insight Recipes to Insight-live
Importing Generic CSV Recipe Data into Insight-Live
Insight-Live Meets a Silica Deprived Glaze Recipe
Insight-Live Quick Tour
Liner Glazing a Stoneware Mug
Make a precision plaster mold for slip casting using Fusion 360 and 3D Printing
Making ceramic glaze flow test balls
Making test bars for the SHAB, LDW and DFAC tests
Manually program your kiln or suffer glaze defects!
Mica and Feldspar Mine of MGK Minerals
Predicting Glaze Durability by Chemistry in Insight-Live
Preparing Pictures for Insight-live
Replace Lithium Carbonate With Lithium Frit Using Insight-Live
Replacing 10% Gerstley Borate in a clear glaze
Same Beer Bottle Mold Using Fusion 360 and OnShape CAD
Signing Up at Insight-live.com
Signing-In at Insight-live.com
Slip cast a stoneware beer bottle
Substitute Ferro Frit 3134 For Another Frit
Substituting Custer Feldspar for Another in a Cone 10R Glaze Recipe
Thixotropy and How to Gel a Ceramic Glaze
Use Insight-live to substitute materials in a recipe
Watch Thixotropy Happen With a 20kg Batch of Dipping Glaze
Create a Synthetic Feldspar in Insight-Live
A step-by-step of how to duplicate the chemistry of Minspar by mixing other materials. You will learn the calculate process, the type of testing to do and how to keep track of the results with notes, pictures and links.
You can also watch this at Screencast-o-matic.com
Transcript/Notes
First, let's see how to do the chemistry part.
In my Insight-Live account, I have made a recipe with only Minspar, I'll open it.
Let's look at the chemistry of Minspar (click on it), it has 6.5-to-4 of sodium vs. potassium.
In the Materials Manager I with search "nepheline|custer" (to show both).
Clicking on each to show their chemistry makes it evident that Custer feldspar has the reverse sodium/potassium (K:Na) ratio of Minspar and Nepheline has a little more Na2O but a lot less SiO2.
more..
So it seems a blend of Custer and Nepheline could work, the latter diluting the amount of K2O in Custer down to what Minspar has. However in doing that I will under-shoot the Na2O, I will need an auxiliary concentrated source of Na2O.I create a new recipe with three lines, Nepheline (50 parts), Custer (50 parts) and Ferro Frit 3110 (zero parts). I will name the recipe and give it a code number (that code number links fired tests with their Insight-live records). Click "Done Editing", turn on the formula calculation for this recipe and assess.
This initial guess-recipe produces too much K2O and not enough Na2O.
Turn on Calculation Mode with an increment of 5.
Reduce Custer and increase Nepheline and try to match the K2O and Na2O. It becomes evident that extra Na2O is needed: Add 5 of the frit. Then 10.
As we zero-in it becomes evident a little CaO is needed: Add 1 Calcium Carbonate. Then 1.5%.
At 60 Nepheline, 16 Custer, 6 Frit 3110 and 1.5 whiting we have all the fluxes matched.
The Al2O3 and SiO2 need to be increased so add kaolin and silica to the recipe.
Nudge up the kaolin until Al2O3 matches, the silica until SiO2 matches.
Retotal the recipe to 100.
This is only a small part of the work, now I must start testing.
Notice the work I have done: I entered notes; did a melt flow comparison test comparing how Minspar and this substitute melt at cone 6, I uploaded that picture and annotated it.
Next I tested it in a recipe called "Perfect Clear" - I'll search that and open it to the right. We mixed that recipe using Minspar and the substitute, glazed test tiles and did another side-by-side flow test - carefully documenting the results of all tests with notes and pictures. This gives us an audit trail of all the testing that was done here.
Let's link this L4441B to the Perfect Clear recipe.
It is also possible to find these recipes in our account by searching recent pictures and clicking on one - it shows what recipes it is linked to.
We have a group account at Insight-live, it has 11,000 recipes that Joe Schmidt I have done tests on in the last 40 years! Everything we have done is recorded with notes, pictures, data and calculations.
Links
| Media |
Substitute Ferro Frit 3134 For Another Frit
I use my Insight-live account to do the glaze chemistry to replace Ferro frit 3134 with combinations of three other common Ferro frits. We will see the challenges of doing this in three different types of recipes. |
Calculating a substitute for Minspar
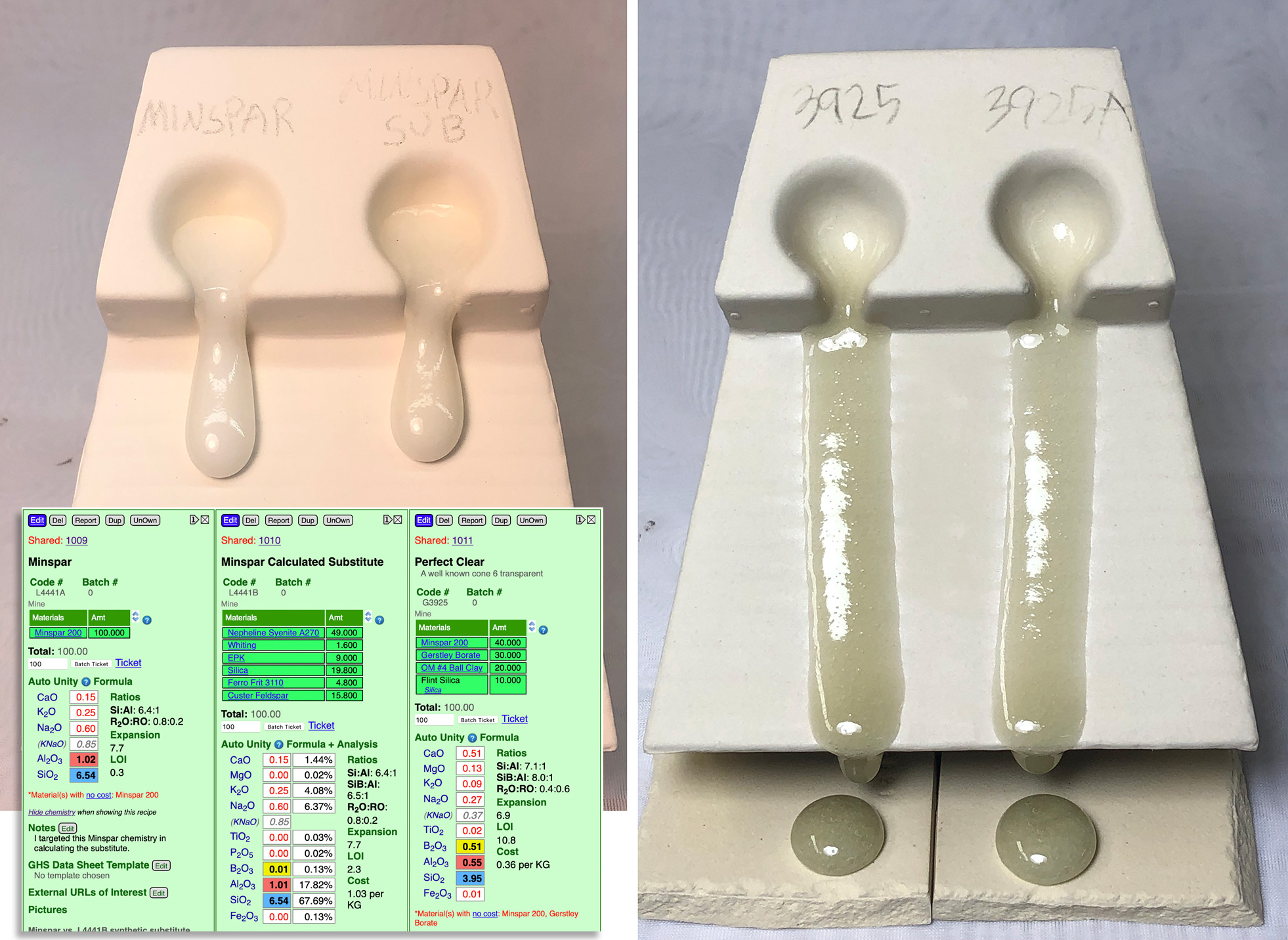
This picture has its own page with more detail, click here to see it.
Why do this? We did not have it in stock and customers needed to mix recipes. When the chemistries of the two feldspars are very similar substitution is often not a problem, especially when a recipe only calls for 5 or 10%. However, when a recipe calls for a significant percentage the situation becomes much trickier (in our cone 6 test recipe, "Perfect Clear", 40% Minspar is needed). Feldspars are almost a glaze in themselves, just needing silica and alumina to shift their chemistry toward 'glazedom'. In this project I calculated a mix of materials, in my Insight-live.com account, that sources the same chemistry as Minspar. I made cone 6 GLFL tests comparing the pure Minspar and Minspar substitute (left) and comparing the Perfect Clear glaze with each feldspar (right). As you can see, the similarity in melt flow is stunning! This is a good demonstration of just how practical and valuable glaze chemistry calculation can be.
Click here for case-studies of Insight-Live fixing problems

This picture has its own page with more detail, click here to see it.
You will see examples of replacing unavailable materials (especially frits), fixing various issues (e.g. running, crazing, settling), making them melt more, adjusting matteness, etc. Insight-Live has an extensive help system (the round blue icon on the left) that also deals with fixing real-world problems and understanding glazes and clay bodies.
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
