| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
Minspar 200
Description: Soda Feldspar
| Oxide | Analysis | Formula | Tolerance |
|---|---|---|---|
| CaO | 1.50% | 0.15 | |
| K2O | 4.10% | 0.25 | |
| Na2O | 6.50% | 0.60 | |
| Al2O3 | 18.20% | 1.02 | |
| SiO2 | 68.80% | 6.54 | |
| Fe2O3 | 0.07% | - | |
| LOI | 0.30% | n/a | |
| Oxide Weight | 566.36 | ||
| Formula Weight | 568.06 | ||
Notes
Formerly called NC-4 Feldspar. Very similar chemistry to the no-longer-available F-4 feldspar.
A 200 mesh flotation-grade soda feldspar from Spruce Pine, NC. Used in the ceramic whiteware industry for sanitary ware, dinnerware, floor and wall tile, artware and glazes.
Physical Properties
===================
Refractive Index: 1.53
Mohs Hardness: 6.0-6.5
Mean Particle Size: 12 microns
Specific Surface Area (sq-m/g): 0.9-1.2
Dry Brightness: 94
Raw color: white
pH: 8.7
% Moisture Content: 0.1
Bulk Density (lb/cu. ft)
Loose: 44
Packed: 70
Bulk Density (kg/cu. meter)
Loose: 7.5
Packed: 1121
Particle Size: 100 140 170 200 325
% Retained tr tr 0.1 0.3 3.6
U.S. Sieve Series
*This is from a data sheet from Jan 2013
Related Information
Minspar 200 original container bag - front and back

This picture has its own page with more detail, click here to see it.
Old Blend Feldspar. What is that?

This picture has its own page with more detail, click here to see it.
Laguna Clay sells a substitute for the no-longer-available G200 feldspar. G200 HP is higher in K2O and lower in CaO than G200, Minspar is an ideal addition since it's K2O is much lower and CaO much higher. A 7:3 G200HP:Minspar mix produces a chemistry that is remarkably close (on paper) to G200. They label this blend "Old Blend". They also list a product called "New Potash" in their pricelist, that is G200 HP.
Which is the champion melter of American/Canadian feldspars?
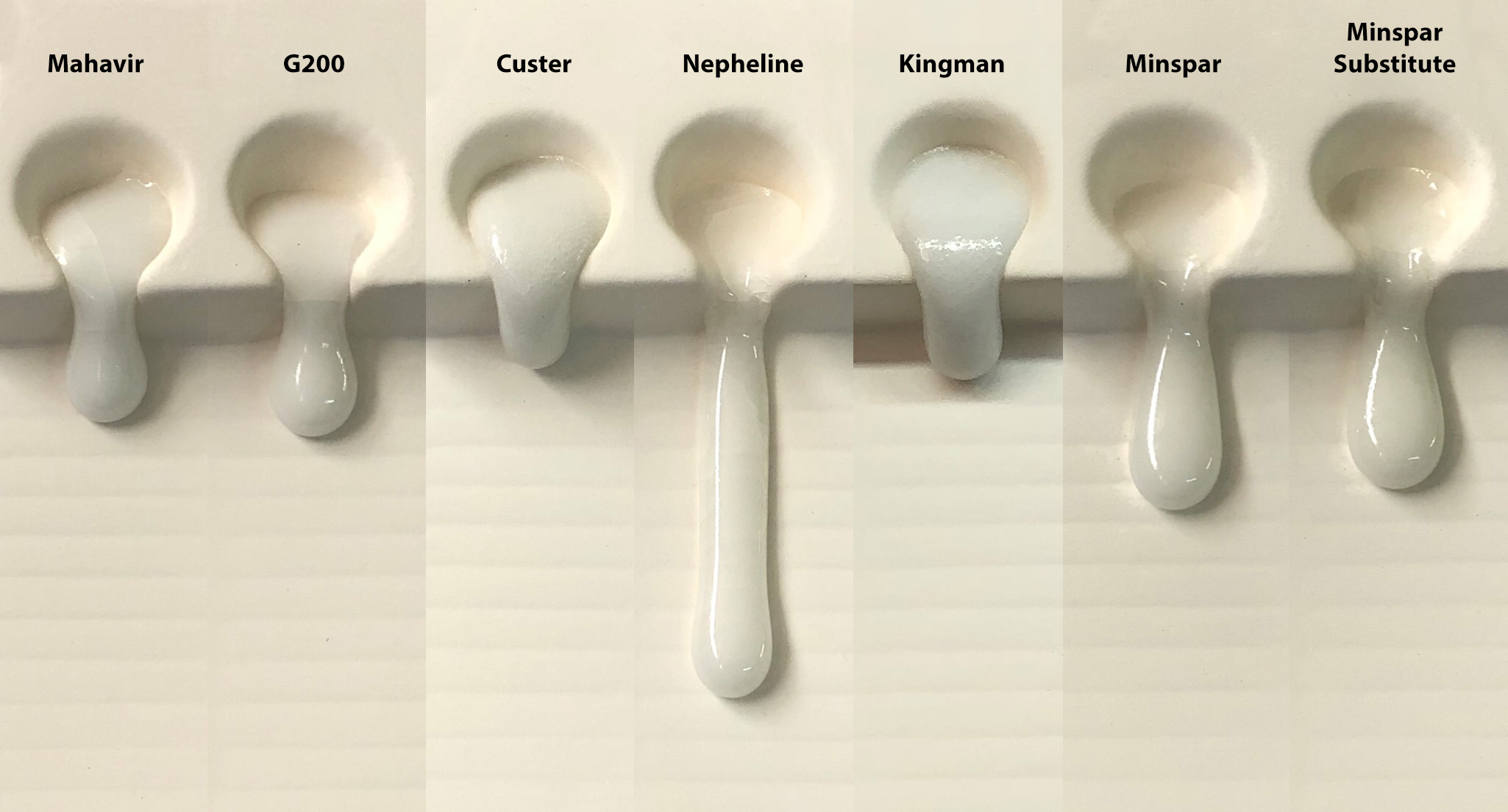
This picture has its own page with more detail, click here to see it.
Feldspars are employed in glaze recipes as melters. So comparing their melt fluidities should be helpful in deciding if one can substitute for another (of course, if possible, a soda-predominant feldspar should be substituted for another soda spar). Feldspars don't melt alone at cone 6 (2200F) so we mixed each with 15% Ferro Frit 3195 (we consider a feldspar a material that sources KNaO). Nepheline Syenite, this is A270, is the champion melter here. Other similar ones can be spotted easily. In the end, degree of melt is a valid consideration in determining if one feldspar is a viable substitute for another in a recipe. Even if the feldspar you want to substitute does not melt as much a little frit can be added to the recipe to make up for the difference (e.g. 3-5%).
This feldspar melts by itself to be a glaze, but crazes badly

This picture has its own page with more detail, click here to see it.
Pure MinSpar feldspar fired at cone 6 on Plainsman M370 porcelain. Although it is melting, the crazing is extreme! And expected. Feldspars contain a high percentage of K2O and Na2O (KNaO), these two oxides have the highest thermal expansion of any oxide. By far! Thus, glazes high in feldspar (e.g. 50%) are likely to craze. Using a little glaze chemistry, it is often possible to substitute some of the KNaO for another fluxing oxide having a lower thermal expansion.
Calculating a substitute for Minspar
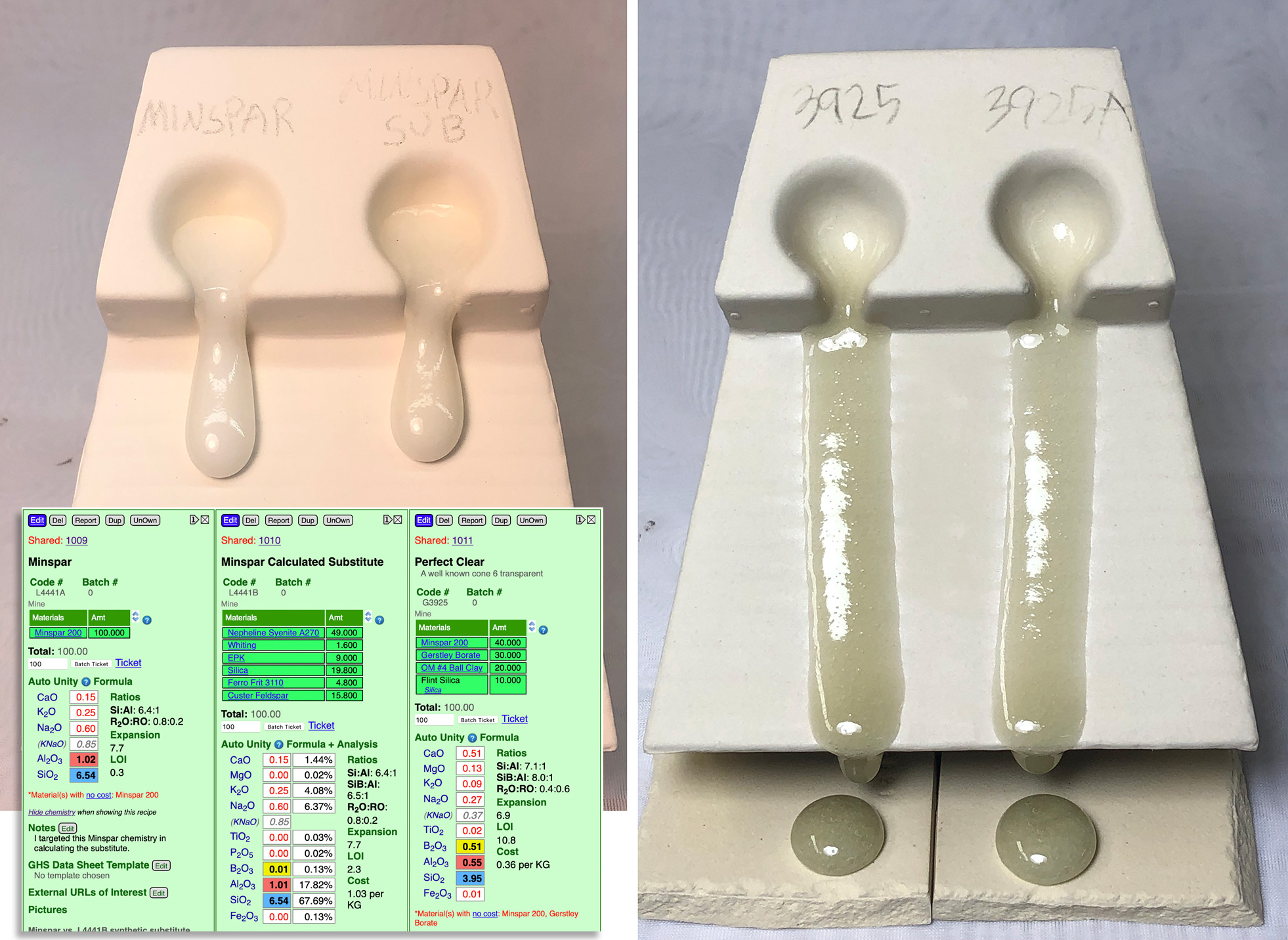
This picture has its own page with more detail, click here to see it.
Why do this? We did not have it in stock and customers needed to mix recipes. When the chemistries of the two feldspars are very similar substitution is often not a problem, especially when a recipe only calls for 5 or 10%. However, when a recipe calls for a significant percentage the situation becomes much trickier (in our cone 6 test recipe, "Perfect Clear", 40% Minspar is needed). Feldspars are almost a glaze in themselves, just needing silica and alumina to shift their chemistry toward 'glazedom'. In this project I calculated a mix of materials, in my Insight-live.com account, that sources the same chemistry as Minspar. I made cone 6 GLFL tests comparing the pure Minspar and Minspar substitute (left) and comparing the Perfect Clear glaze with each feldspar (right). As you can see, the similarity in melt flow is stunning! This is a good demonstration of just how practical and valuable glaze chemistry calculation can be.
A soda feldspar applied like at glaze at cone 4-7

This picture has its own page with more detail, click here to see it.
This is pure soda feldspar (Minspar 200) fired like a glaze at cone 4, 5, 6 and 7 on porcelainous stoneware tiles. The bottom samples are balls that have melted down at cone 7 and 8 (although they appear to be well melted they exhibit no movement in a melt fluidity test. Notice there is no melting at all at cone 4. Also, serious crazing is highlighted with ink on the cone 6 sample (it is also happening at cone 5 and 7). Feldspars have high KNaO, that means they have high thermal expansions. That is why high-feldspar glazes almost always craze. Since feldspar is barely melting at cone 6 it is not possible to make a functional cone 6 glaze that only uses feldspar as the flux (boron, lithia or zinc are also needed).
Links
| Typecodes |
Feldspar
The most common source of fluxes for high and medium temperature glazes and bodies. |
| Materials |
NC-4 Feldspar
|
| Materials |
F-4 Feldspar
|
| Materials |
G-200 Feldspar
|
| Materials |
G200 HP Feldspar
|
| Materials |
Feldspar
In ceramics, feldspars are used in glazes and clay bodies. They vitrify stonewares and porcelains. They supply KNaO flux to glazes to help them melt. |
| Materials |
Kona F-4 Feldspar
|
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
