| Monthly Tech-Tip | Feb 14-15, 2026 - Major Server Upgrade Done | No tracking! No ads! |
A cereal bowl jigger mold made using 3D printing
Beer Bottle Master Mold via 3D Printing
Better porosity for Brown Sugar Savers
Build a kiln monitoring device
Celebration Project
Coffee Mug Slip Casting Mold via 3D Printing
Comparing the Melt Fluidity of 16 Frits
Cookie Cutting clay with 3D printed cutters
Evaluating a clay's suitability for use in pottery
Make a mold for 4-gallon stackable calciners
Make Your Own Pyrometric Cones
Make your own sieve shaker to process ceramic slurries
Making a high quality ceramic tile
Making a Plaster Table
Making Bricks
Making our own kiln posts using a hand extruder
Medalta Ball Pitcher Slip Casting Mold via 3D Printing
Medalta Jug Master Mold Development
Mold Natches
Mother Nature's Porcelain - Plainsman 3B
Mug Handle Casting
Nursery plant pot mold via 3D printing
Pie-Crust Mug-Making Method
Plainsman 3D, Mother Nature's Porcelain/Stoneware
Project to Document a Shimpo Jiggering Attachment
Roll, Cut, Pull, Attach Handle-making Method
Slurry Mixing and Dewatering Your Own Clay Body
Testing a New Load of EP Kaolin
Using milk as a glaze
Comparing the Melt Fluidity of 16 Frits
This project is hopefully going to teach me something about how frits melt over ranges of temperatures, how they compare in melting, how that relates to their chemistries and which ones melt in a way that makes them better candidates as body frits, balanced glazes on their own or as a special-purpose additives to glazes.
I mixed 100g of each of the frits with 3.25g of Veegum to make them plastic. I shook the powders in a bag and then I stirred it into 35 grams of water in a cup. When they got to dry mud consistency I hand-kneaded them to a smooth consistency. I divided the batches into 12 gram pieces and rolled each into a ball for drying. That produced dried balls about 9 grams each.
In the tests done so far (up until early January 2020) it is evident that most fruits have completely done their thing before the kiln even reaches bisque temperature.
Related Information
About to start a melt-flow comparison of 16 different frits

This picture has its own page with more detail, click here to see it.
I am going to fire them at about 10 temperatures starting at cone 022. These will be a great to determine whether they suddenly melt or slowly soften over a range of temperatures. I will be able to see how wide that range is and how they behave as they go beyond it. I also included Gerstley Borate, mother nature's nature boron source. They way it melts should clear contrast with the frits.
VeeGum and CMC gum can plastify non-plastic powders for making melt-flow test balls

This picture has its own page with more detail, click here to see it.
Fluxes used in ceramics are almost always non-plastic, they cannot be formed like clay - frits and feldspars are good examples. And they don't dry hard. To make balls for use in the GBMF test for melt flow a binder needs to be added. Traditionally we have used Veegum, however, it interacts with materials enough to affect melting - CMC gum does not. That being said, Veegum dries better (these balls can dry very slowly). When simply comparing the melt of two materials either is fine.
Each ceramic powder responds differently to being water-mixed with a gum or plasticizer. Some material-gum mixes uptake water so well that it can be worked in drops at a time until a plastic material is produced. Others require vigorous mixing into a slurry and then dewatering on a plaster surface. We target 9g balls, they fit into the reservoir of the melt flow tester. To make one ball we start with 11 or 12g of powder (to allow for waste) and then form them into 12g (wet) balls - these dry them down to the 9g weight. We are almost always comparing the flow of two materials, in these cases it is only important that the two balls be the same weight - so we trim the heavier one down to the weight of the lighter one. Does cornstarch work? No, the mix is not plastic. Psyllium? Yes, but it has a flakey texture and demands more water.
If you are testing a plastic material then a binder is not needed.
Frit melt fluidity comparison - 1300F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1300F and held for 15 minutes. Some are still burning off carbon (which seems strange). There are two early leaders: Ferro frit 3110 and Fusion frit F75 are starting to deform (they have almost the same chemistry). Amazingly, these two frits have low boron, they rely on high soda as the flux.
Melt fluidity comparison of frits - 1350F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1350F and held for 15 minutes. Some are still burning off carbon (which seems strange). The two FZ16s are starting to move. Frit 3134 is expanding. 3602 is also starting to melt.
Melt fluidity comparison of frits - 1400F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1400F and held for 15 minutes. Frit 3134 is still expanding. 3602 is also starting to flow. A number of them are shrinking and densifying like a porcelain would.
Melt fluidity comparison of frits - 1450F
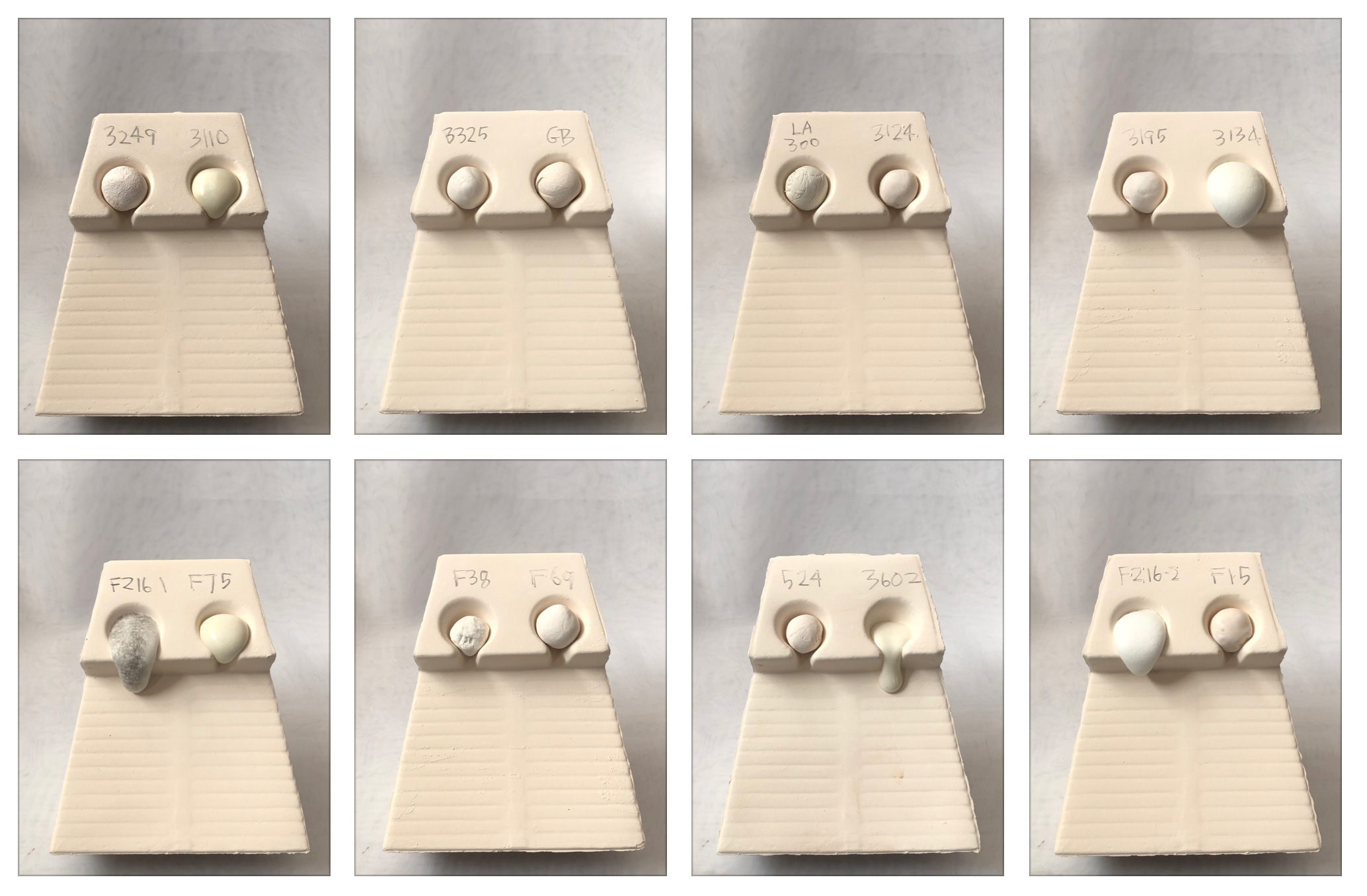
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1450F and held for 15 minutes. Frit 3134 is still expanding. 3602 is blasting out of the gate, taking the lead. F75 is starting to flow.
Melt fluidity comparison of frits - 1500F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1500F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are really starting to move. 3195, F38 and F15 are softening.
Melt fluidity comparison of frits - 1550F
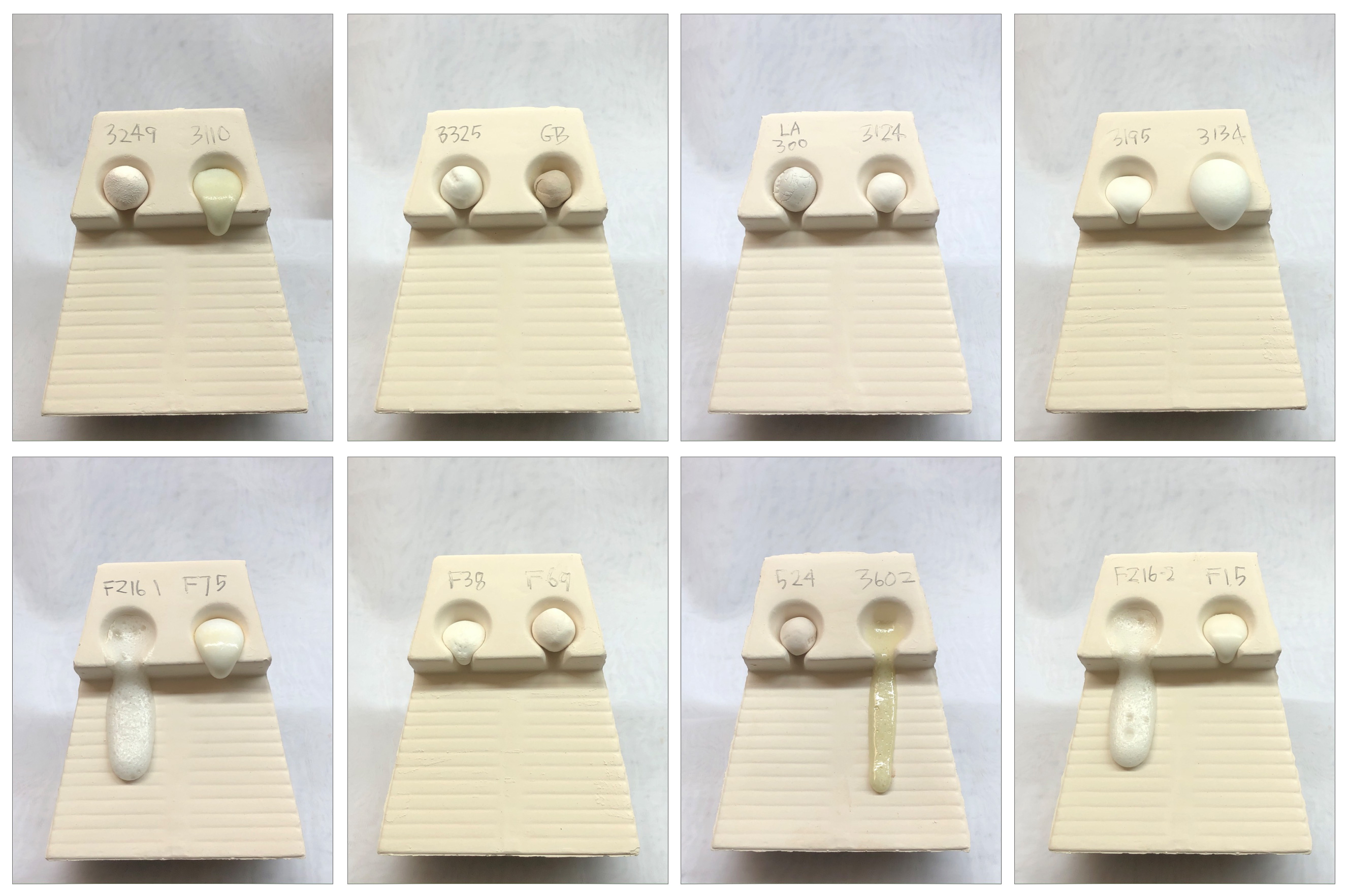
This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1550F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are going to be off-ramp by next firing.
Melt fluidity comparison - 1600F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1600F and held for 15 minutes. Frit 3134 is still expanding. 3602 and FZ16 are off-ramp. F-75 is starting to flow. Gerstley Borate has suddenly shrunk. Frit 3110 is demonstrating its wide range, slowly softening more at each temperature.
Melt fluidity comparison of frits - 1650F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1650F and held for 15 minutes. FZ16 has turned crystal clear and spread out across the runway (has low surface tension). Frit 3110 has so much surface tension that the flow can be lifted off the tester. Since 1600F Gerstley Borate has gone from unmelted to passing all the rest!
Melt fluidity comparison of frits - 1700F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1700F and held for 15 minutes. 3110 is finally starting to move. 3134 also (being full of bubbles). Gerstley Borate has turned almost transparent (because the Colemanite portion of it is now melting). 3195 is looking very well behaved compared to most others, forming a bubble free glass of high surface tension (F15 and F524 are starting to do the same).
Melt fluidity comparison - 1750F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1750F and held for 15 minutes. Frit 3110 has taken off. And F75, 3195 and 3134 (the latter two having big differences in surface tension).
Frit Melt Fluidity Comparison - 1800F

This picture has its own page with more detail, click here to see it.
Fired at 350F/hr to 1800F and held for 15 minutes (I already did firings from 1300F-1750F in 50 degree increments, all of them are visible in the parent project). Frit 3110, 3134, 3195, F75 have run all the way down. All of the frits have softened and melted slowly over a range of temperatures (hundreds of degrees). By contrast, Gerstley Borate, the only raw material here, suddenly melted and flowed right over the cliff (between 1600 and1650)! But not before Frit 3602 and FZ16 had done so earlier. Frit 3249 is just starting to soften but F69 (the Fusion Frits equivalent) is a little ahead of it. LA300 and Frit 3124 are starting also. F524, F38, F15 will all be over the end by the next firing. The melt surface tension is evident by the way in which the melts spread out or hold together.
Frit Melt Fluidity Comparison - 1850F

This picture has its own page with more detail, click here to see it.
These melt flow tests were fired at 350F/hr to 1850F and held for 15 minutes (I did firings at 50-degree increments across a wide range). It is amazing how active some frits are, even well below normal bisque temperatures! Frit 3110, Frit 3134, Frit 3195, Frit F-75 have all flowed all the way down for many previous temperatures. LA300 and Frit 3124 were just starting at 1800F, look at them now! Frit F-524 and Frit F-38 have gone from half-way at 1800F to water-falling over the end. Frit 3249 is still not out-of-the-gate but Frit F-69 (the Fusion Frits equivalent of 3249) is half-way. Note how the melt surface tension is evident by the way in which the melts spread out or hold together. By contrast, Gerstley Borate (labelled "GB"), the only raw material here, suddenly melted and flowed right over-the-cliff between 1600 and 1650! The best melter of all of them is high-boron high-zinc Frit FZ-16.
Fusion Frit FZ-16 melt flow over many temperatures

This picture has its own page with more detail, click here to see it.
This melt flow tester demonstrates the beautiful crystal-clear glass this zinc frit creates by 1700F. It fits this porcelain without crazing, even though it is very thick and high in sodium (the high zinc and boron are countering it to keep the thermal expansion down). It runs off the end of the runway around 1600F on this GLFL test, rivalling lead bisilicate. This is a more concentrated boron source than even Gerstley Borate. Everything about this material screams “ultra gloss”. What a material to build a fluid-melt reactive super-glaze on!
Ferro Frit 3602 melt flow over many temperatures

This picture has its own page with more detail, click here to see it.
This demonstrates the amazing melt behaviour of lead-as-a-flux for ceramic glazes. Not only does it melt early, but it softens slowly over a 300F range of temperatures before it goes off the end of the runway on this GLFL test. Then, when fired 200F hotter than that, it remains a stable, clear and uncrazed glass. Beginning around 1750F, this becomes a transparent glaze, by itself.
Frit melt runoff winner

This picture has its own page with more detail, click here to see it.
By 1700F the Fusion zinc frit FZ-16 has already established itself as a better melter than lead bisilicate.
Links
| Materials |
Frit
Frits are made by melting mixes of raw materials, quenching the melt in water, grinding the pebbles into a powder. Frits have chemistries raw materials cannot. |
| Glossary |
Frit
Frits are used in ceramic glazes for a wide range of reasons. They are man-made glass powders of controlled chemistry with many advantages over raw materials. |
| By Tony Hansen Follow me on        |  |
Got a Question?
Buy me a coffee and we can talk

https://digitalfire.com, All Rights Reserved
Privacy Policy
