
Cone 6 Iron Reds | Matte Bases | Glossy Bases | Honey Transparents | Floating Blues | PV Clay - Cone 06 Raku | LowFire Clear
Honey/Amber Gerstley Borate Cone 6 Glazes
These pages were a study of how we substituted Boraq 1, Boraq 2 and Boraq 3 into common recipes.
Boraq was developed by Plainsman Clays as a substitute for Gerstley Borate (under code number L3127E).
The development effort took place during the early 2000s, the initial period when the demise of Gerstley Borate appeared imminent.
Other companies, including Laguna Clays, introduced similar products at the time.
Later Laguna Clays began processing a last stockpile of the material they found at the mine and interest in substitutes waned.
In 2023 the cycle appears set to repeat so these pages are pertinent again.
Gerstley Borate was used to create fluid melt glazes at cone 4-6 to host the
development of transparent colors and crystalization. Yellow colors can be achieved adding just rutile (up to 5%)
and these can be darkened with iron oxide (fluid transparent boron glazes
can support significant amounts of iron and yet still be quite light in color).
Very small amounts of lithium can have a remarkable effect on rutile iron
versions, transforming them into a much more interesting variegated surface.
Of course leaching and hardness issues could arise with this type of glaze.
This is a variation on the 50:20:30 recipe (with 50 GB along with 15-18 kaolin and 32-35 silica) with
3-4% iron and rutile for the color and variegation.
Since this glaze is so fluid, darkly colored versions of it work well as
overglazes on lighter colored more melt-stable mattes.
Conclusions from Feedback
|
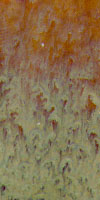
The upper two cone 5 porcelain tiles show the maximum difference we have been able to achieve
using Boraq 1 for GB (most are a much better match). This variation is "tweaked" with the addition of a little
lithium to variegate it more than the rutile:iron mix will do alone. While the Boraq 1 glaze is more
interesting, its darker color is curious. The GB version goes on much thinner
and takes longer to dry.
The chart shown here compares the mole% formula of the Gerstley Borate version of the glaze with the Boraq
versions.
An interesting comment: "I would think the Mg and Ca would have a bleaching and "greening" effect on the
iron in this glaze.
Dropping the Al2O3 and B2O3 content may help to bring
about a more generalized opacity helping to lighten the colour." This supports the
idea of dropping the amount of Boraq 1. However notice the 80% Boraq 1 sample.
It is much darker. Also notice that the Boraq 2 version supplies plenty
of CaO and MgO and not as much boron. It is almost identical.
|
| G2826A | GB Mole% | Boraq1
Mole% | 80% as much Boraq1 | Boraq2
Mole% |
| CaO |
13.99 |
11.27 |
8.83 |
15.01 |
| Li2O |
0.97 |
0.97 |
0.95 |
0.96 |
| MgO |
3.11 |
1.93 |
1.52 |
1.71 |
| K2O |
0.14 |
0.26 |
0.22 |
0.23 |
| Na2O |
2.60 |
1.97 |
1.55 |
1.75 |
| TiO2 |
3.57 |
3.56 |
3.49 |
3.55 |
| Al2O3 |
4.97 |
5.47 |
6.07 |
5.35 |
| B2O3 |
14.81 |
17.52 |
13.70 |
15.53 |
| SiO2 |
53.95 |
55.09 |
61.76 |
53.98 |
| Fe2O3 |
1.84 |
1.83 |
1.80 |
1.82 |
We have removed a couple of oxides showing trace amounts to reduce clutter.
Also note that the last digit of precision is not significant.
Click here to learn about Mole% calculations.
|

With GB

With Boraq3
|
With Boraq1

With 80% as
much Boraq1
|
This glaze is really quite puzzling. Although we have a good match with Boraq 2
and Boraq 3 it would be good to understand what is happening with the others. All of these
are white porcelain tiles. Do you have any idea why the 80% Boraq 1 is so dark?
Here is an interesting comment from Rick Shanks: Even though the analysis
indicates little difference in CaO:MgO ratios, I think if you do a line blend
that increases MgO and decreases CaO you will find browner iron colors as MgO
increases in relation to CaO, regardless of total MgO+CaO. Eliminating CaO as
much as raw material choises will allow for the high MgO end member and
eliminating MgO as much as raw material choices will allow for the high CaO end
member and also trying to match flow of both end members and reference GB glaze
would constitute a fair and revealing evaluation using the line blend method. Of
course, the higher the SiO2:Al2O3 ratio the more phase separation or milky white
opalescence. This will opacify/whiten the color but all things being equal
higher MgO:CaO ratio will give browner iron color. If the SiO2:Al2O3 ratio is
high enough (even without changing total refractory / SIO2+Al2O3), the glazes
will be nearly white or solid opaque phase. Also the phase separation of the
"80% as much Boraq1" isn't flowing or floating downward as much as the
other samples, indicating less flow or fluidity. Fluidity is one of the most
important variables to consider or reference standards to measure against when
trying to duplicate effects with the "marginal" glazes that potter's
envy.
HTML Recipe report from INSIGHT 5.3
2826A1
Butterscotch with GB |
2826A2
Butterscotch with Boraq 3 |
|
|

|
|

|
By Tony Hansen
Follow me on
                        |  |















